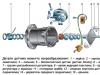
Each motorist knows how important the correct operation of the battery is the functioning of the entire mechanism. It is lead-acid batteries that are used as starter cars for passenger cars.
In this article, we will tell about the device and the principle of operation of the AKB, also it will be about the diagnosis of the battery, the most common problems and the methods of its recovery.
The device and the principle of operation of the AKB
The body of the product is expected from propylene, this material was selected for two main reasons:
- Does not spend current
- Not destroyed by acid
One device includes six batteries interconnected. A separate battery combines negative and positive electrodes (a lead alloy is taken for their manufacture, for negative electrodes - lead-calcium composition), filled with active mass.

The insulation of the opposite charge layers provides a separator from plastics. In order to improve corrosion resistance, lead-calcium alloy for electrodes can be diluted with silver or tin.
The active mass of negative electrodes consists of spongy lead, positive - from lead dioxide.
There are two types of battery:
- With liquid electrolyte.
- SO special Material, pre-impregnated with non-long electrolyte.
Today, batteries with a liquid electrolyte are most common.
The principle of operation is based on the transformation electrical Energy In the chemical during charge, during the discharge there is an opposite effect - chemical energy is converted into electrical.
The battery discharge occurs as a result of the connection of consumers: the active mass of the electrodes (negative and positive) enters into interaction with the electrolyte.

As a result, lead sulfate with water and the level of electrolyte density drops. With the proper operation of the generator, it gives charging the battery during engine operation.
Also, the battery can also be charged with a special device, as a result of the charge, lead sulfate and water turn into lead, lead dioxide and sulfuric acid, thus the density level increases.
Note! The charge must be performed taking into account the recommended electrical stress, in case of violation this rule operation, the service life of the device can be significantly less than the specified one.
As a result of high voltage, the level of electrolyte falls, low voltage It may cause an incomplete charge of the battery. In general, the battery life is about five years, it all depends on the conditions in which the device is operated.
Instrument parameters:
- Rated container. This indicator is measured in amps-hours (Ah) depends on the energy of the charged device during the discharge (20 hours). For example, the device at 50 ACC for twenty-hours gives current 2.5 A.
- Rated voltage consists of a voltage of individual batteries, a passenger car is 12 V.
- Cold scrolling current indicates the ability of a car to start in a cold period. The higher the indicator, the easier the engine to start in the frost.
Akb faults
The battery, like any mechanism, may fail, as a result of which it will not work incorrectly either stop working at all. Below we will look at the most frequent problems in the work of the system and teach how to fix them.

Very often, car owners face the problem of the oxidation of the conclusions, as a result of which the current supply is stopped and the opposition increases in the chain, thus the entire electrical system comes out.
To solve the problem you need:
- Remove terminals.
- Clean the terminals and battery conclusions.
- Now we put everything in place, check the correctness and reliability of fasteners - the terminal should not move or depart from the output.
- It is recommended that the terminal is lubricated by technical vaseline.
Many drivers complain about the rapid discharge of the battery.

There may be two reasons for this:
- Pollution of electrolyte located inside the device.
- Contamination of the device itself.
IN this case It is necessary to remove the battery and wipe all the contacts well, pay attention to the device can not be left wet. Next, you need to check the purity and level of electrolyte, if necessary, it is necessary to replace the liquid to the new one.
How to diagnose a car battery

Before you begin the diagnosis of the device, it is necessary to remove it.
Note! The first is removed negative terminal. However, when installing it connects the latter.
Electrolyte level
Conduct the level and density of the battery solution is recommended for at least once every three months. The level is checked using a glass tube (the inner diameter should be 4-5 mm) through the filler appeals.
The tube should be lowered to the end, the outer prompts must be fine with your finger and remove. The permissible level of electrolyte in the battery must be 12-15 mm.
If there is a tube in the battery, the level may exceed 3-5 mm.
Electrolyte density
The second indicator is the electrolyte density - plays an equally important role, so it is also necessary to control it.
During operation, the fluid density can flucta fluid, full discharge - full charge, indicators may vary by 0.15-0.16 units.
The high level of density can cause a rapid wear of the instrument, with a low density level, the engine start will be performed for a long time and problematic.
Battery charge level
To check the charge of the car battery should use load plug. This device It has a voltmeter, switch loading resistance, handle and two contacts.
Also, the charge can be determined by repaying from the output voltage, for this you will need a multimeter and a voltmeter (it is important to turn off the minus terminal).
Modern devices are equipped with an indicator showing the battery charge. If the device is charged, then the indicator is on green, discharged - white or red.
To charge car battery It is necessary to use a charger, which is a source of current: positive contact with a plus terminal, negative - to a minus.
Methods for recovering battery
Each motorist is interested in how to extend the battery life or how to restore it.

And yet, if you missed or ignored some tips regarding the operation of the device, we should not despair, below we will tell you what ways to restore the functioning of this device.
Using CTC
KTC (Training Cycle), this procedure helps to restore the capacity and avoidance of the sulfate process. The CTC procedure consists of several stages of discharge and battery charge.
For this we will need:
- Charger.
- The voltage control device is a voltmeter.
- The device for monitoring the level of electrolyte density is a carometer.
- Light bulb.
So, first fully charge the battery. It is important that while charging a cover with cans were removed. The battery should be charged from 6 to 8 hours.
Upon completion of the procedure using the area, it is necessary to check the electrolyte density level in each bank separately - the indicator should be 1.27 g / cm. cube If necessary, distilled water is added to the banks or sulfuric acid, after which the battery is postponed for another half an hour.
Multiple charging mode
A no less simple method for restoring the correct operation of the battery, proposed by car specialists, is to carry out several stages of the charge of the device with interruptions. Initially, it is necessary to set the current level to the indicator 0.04 of the nominal battery volume. After 8 hours of charging, you must make a 12-hour break (not more than 16 hours).
The break is necessary for aligning the inner potential and external lead plates, the diffusion of dense electrolyte is performed in the intervals between the electrodes.
After the break, the battery charge procedure is renewed. It is recommended to carry out at least 5 such procedures. During the increase in volume, the level of electrolyte density will increase, as a result of which it must be diluted with distilled water and control the level indicator, it is important to keep it within the normal range.
Chemicals
So, first you need to complete a complete charge. rechargeable device, after which it is important to drain the entire electrolyte. Now it is necessary to wash the container with distilled water, at least three times.
For next stage Flushing We take a solution of 5% (weight) ammonia and 2% (weight) of the trillion V. Pour it into the tank pre-purified with distilled water, with which the electrolyte was pulled out and leave for an hour.

The spray and active gas release will be observed, this is the process of desulfation. Upon completion of the gas division, the procedure can be considered complete. Now we drain the liquid from the battery and rinse the container again using distilled water (2-3 times). Now fill the battery with a new electrolyte and spend a complete charge.
If strong sulfate is observed, then the recovery of the battery can be spent a couple of times. However, we note that such a solution cannot be prepared independently, it is recommended to contact a specialist.
Pulsed current
This method will help solve the problem of closure in a battery bank, many do not know about such a method or do not risk it to use, however, according to the reviews of many motorists, it is safe to say that the burning method with a pulmonary current is quite effective.
Connect the battery to the source produced by the high current (in this case it is at least 100 amps). Very often, a welding machine is used with such a goal. The closure in the bank is burned as a result of a two-second passage of this current.

The process of restoring automotive batteries in detail
Like all things, the battery has its limited life. In the case of lead-acid batteries, the restoration of which we will talk, the service life is equal to an average of 3-4 years. Often owners of cars just buy new batteryIf the old starts to bring. In this case, there are malfunctions in which the performance of the battery can be restored and used for some time. Let's consider the main reasons for the output of the battery and the methods of their recovery.
This article will talk about lead-acid acb. About, you can read by reference. Battery malfunctions can be divided into two groups: external and internal.
External
The following are external malfunctions and ways to eliminate them:
- The plastic battery case is damaged. Such damage (crack, hole) can be caused by both external influence and as a result of processes in the Akb itself (bloating, overheating, etc.). Here you need to understand that in the case of large spacing, repair does not follow and better buy a new battery. And if the crack is small, you can close it using plastic and soldering iron. Before carrying out work, the entire electrolyte merges. When the crack is embedded, you need to pour a new electrolyte and charge the battery;
- Accord terminals oxidized. Here, the task of restoration is much easier. It is only necessary to clean the shallow sandpaper and a rag of the layer of oxides. The same operation is not bad on the terminals of the connected wires. After that, you can lubricate the terminals with a small amount of machine oil.



As you can see, among the internal faults, the AKB successfully eliminates sulfate. And if it is not in the running stage. Therefore, we will consider several ways to restore the battery in the case of sulfation of lead plates. But first we will list that we may need to work:
- Fresh electrolyte;
- Distilled water;
- Battery charger;
- Hydrometer (electrolyte density measurement);
- Protective equipment (glasses, gloves);
- Desulphating additive and some other chemicals.
How to restore the car battery during sulfate
To begin, it is necessary to inspect the AKB. To do this, drain the electrolyte and rinse the cans with distilled water. After that, spend a visual inspection. If the plates in the banks are damaged or trembled, then the restoration of such a battery is inappropriate. If there is no external damage, the fresh electrolyte is poured and is carried out (dissoluting lead sulfates on plates).
Restoring a car battery using KTC
CTC is decrypted as a control cycle. Conducting this event helps restore the capacity and eliminate sulfate when it is not launched. The process is a series of battery discharge cycles. In addition to chargerIt will require an areaometer (electrolyte density control), a voltmeter (voltage control) and a light bulb (or other source of consumption).
First, a complete charge of the battery is performed.
Do not forget to remove covers with battery cans! Charging current must be installed 10 percent of the nominal battery capacity. So, for common battery 55 AH, the charge current must be not higher than 5.5 amps.
Charging is carried out 6-8 hours. By the end of the process, the voltage at the terminals increases, and the battery perceives the charge.
At the end of the charging, measure the electrolyte density area in all banks. The electrolyte density in a fully charged battery should be 1.27 g / cm. cube If the density is less or more, then the fraction of sulfuric acid or distilled water, respectively. After dilution, the battery is set to charge for 30 minutes. During this time, the electrolyte is mixed.
Electrolyte density table
The electrolyte density table is given below and the characteristics associated with it:
| Account degree,% | ||||
|---|---|---|---|---|
| Electrolyte density, g / cm. cube (+15 gr. Celsius) | Voltage, in (in the absence of load) | Voltage, in (with a load of 100 a) | Account degree,% | Frozening temperature of electrolyte, gr. Celsius |
| 1,11 | 11,7 | 8,4 | 0 | -7 |
| 1,12 | 11,76 | 8,54 | 6 | -8 |
| 1,13 | 11,82 | 8,68 | 12,56 | -9 |
| 1,14 | 11,88 | 8,84 | 19 | -11 |
| 1,15 | 11,94 | 9 | 25 | -13 |
| 1,16 | 12 | 9,14 | 31 | -14 |
| 1,17 | 12,06 | 9,3 | 37,5 | -16 |
| 1,18 | 12,12 | 9,46 | 44 | -18 |
| 1,19 | 12,18 | 9,6 | 50 | -24 |
| 1,2 | 12,24 | 9,74 | 56 | -27 |
| 1,21 | 12,3 | 9,9 | 62,5 | -32 |
| 1,22 | 12,36 | 10,06 | 69 | -37 |
| 1,23 | 12,42 | 10,2 | 75 | -42 |
| 1,24 | 12,48 | 10,34 | 81 | -46 |
| 1,25 | 12,54 | 10,5 | 87,5 | -50 |
| 1,26 | 12,6 | 10,66 | 94 | -55 |
| 1,27 | 12,66 | 10,8 | 100 | -60 |
After that, you need to discharge the battery. This requires a suitable source of energy consumption. The easiest way to choose a light bulb. Of course, if there is a memory (charger) with a discharge function, you can defuse the battery.
How to calculate the power of the light bulb? We take the value of the current of the current in the amount of 10 percent of the capacity of our battery. That is, at 55 ah it will be 5.5 amps. This value is multiplied by 12 volts and get 66 W. Such power we need a light bulb.
We connect the source of consumption to the battery terminals and leave it until complete discharge, that is, until the voltage decreases to 10.2-10.6 V. Read more about. If the battery did not lose the capacity, then the discharge time during the above parameters will be about ten o'clock. The smaller this time, the greater the loss of the tank.
After discharge, the battery must be immediately charged and so multiple cycles. As a result of the procedure, the sulfate of the plates decreases, the internal resistance of the AKB decreases and the capacity increases. Thus, the recovery of the car battery is performed with a minor sulfate.
Restore car battery in multiple charging mode
The current is set at 0.04 from the rated capacity of the battery. Charge time is about 8 hours. Then there is a break for 12-16 hours. Breaks are made in order to leveled the potentials inside and on the surface of lead plates. In this case, the diffusion of a more dense electrolyte into the space between the electrodes.

Then again starts the battery charging cycle. Such cycles of charge-discharge can be carried out to 5. As the capacity increases, the density of the electrolyte will grow. Control this value and dilute it with distilled water if necessary. We also recommend reading about.
Not every car enthusiast knows how to restore the car battery. And this can extend his life for several years and protect the car owner from unexpected financial expenses. To date, there are four basic ways to extend the operation of any battery.
The battery is responsible for the process of continuous supply of the required voltage. Accordingly, they are an indispensable component of the work of certain mechanisms of machine and its and appliances. Everyone knows that nothing is forever. As a result, each car needs regular technical inspections to identify unfit details. As a rule, any battery (the most common is acidic alkaline and lithium) can be repaired. This option is better than immediately run to the store for a new one.
As for acid-alkaline (they are also called lead-helium), their structure is represented as follows - a pair of plus-minus plates from lead in sulfuric acid. They are most common in the field of automotive industry and in the production of pocket lanterns. Nevertheless, it serves such a battery with respect to long.
The first way to restore the battery is the use of repeated recharging with a short force current. In this case, the charging process should provide temporary segments between recharging. Thus, starting from the first recharging and ending with the latter, the voltage located in the battery is gradually growing and as a result - it will stop perceiving the charge itself.
Pauses are necessary in order for the potentials of the electrodes that are in the depths of the mass of the plates themselves and on their surface, they leveled, which makes restoring more secure. In parallel, the most dense electrolyte begins to leak directly from the pores of the plates themselves into the space located between the electrodes.
Together with the cyclic charge, and together with the increasing capacity of the battery, the density of the electrolyte itself also rises. It is necessary to wait for the moment when the section voltage is equal to two and a half volt, and the density indicator will reach the normal level. Only then the car battery must "relax." Such a cycle should be repeated up to eight times. Also, the charge current itself should be exactly ten times less than the capacity of the chargeable battery.
Replacing electrolyte
Direct recovery of the battery can be carried out with the method of replacement. To do this, you need to completely merge the electrolyte, and then rinse the battery thoroughly hot water. After that, three teaspoons of ordinary soda will be required, which need to be diluted in a hundred milliliters of water.  The resulting liquid must be boiled, pour instead of electrolyte, and after twenty minutes - to merge. Such an action must be repeated several times, followed by a three-time washing of the same hot water.
The resulting liquid must be boiled, pour instead of electrolyte, and after twenty minutes - to merge. Such an action must be repeated several times, followed by a three-time washing of the same hot water.
This method is great for cars batteries. The last stage of this process ends with the infusion of a new electrolyte and a daily charge. After that, the battery charge for six hours to ten days in a row. The charger must have the following characteristics - the voltage is not more than sixteen volts, but not less than fourteen, and the current is no more than ten amps.
Reverse charging
Recovery with reverse charging is also possible. However, this method provides for a fairly powerful source of the voltage itself (the same welding machine). It must have a tension of at least twenty volts with a current of at least eighty amp. When you need the required device, the next step becomes the opening of the jams of cans and their inverse charge. To carry out such charging, you need to attach a plus charging device To the "minus" battery, and to its "plus" - "minus" of the charging device. It can extend his life for several years and protect the car owner from unexpected financial expenses. 
During the charging process, the battery will boil, but nothing terrible. The charging itself should last no less and no more than half an hour, after which the old electrolyte merges, the container is washed with hot water and only then you can pour a new electrolyte. The next action becomes the use of another charging device with a current of up to fifteen amps. They charge the battery a whole day.
Distribution of charge in distilled water
Using the last, fourth method, the battery is realistic to restore in less than one hour. If it is completely discharged, it should be previously charged. After that, the electrolyte is also completely drained and washed several times with water. Next, it is necessary to pour the battery capacity of an ammonium type, which includes two percent of the same trilder and five percent of the ammonia itself, it is necessary to pour into the washed capacity of the battery. When it is assisted, the process of so-called desulfation is carried out, which lasts up to one hour. During this process, the characteristic isolation of the gas and the occurrence of small splashes on the surface of the inflighted solution occurs.
After all of the above, it is necessary to rinse the battery several times with a simple distilled water, followed by the influence of electrolyte acceptable density. Then the battery is charged and it can be considered completely renovated. By summing up, we can say that, in general, the restoration of the car battery can be considered not very difficult.
I can confidently say that the cessation of gas is evidenced by the completion of desulfation. If the sulfate is too strong, then it is necessary to repeat the processing process to restore the battery fully.
Video "How to restore the capacity of an old battery"
The record shows a method for charging a lead battery at home.
How to restore the performance of a car battery
Battery capacity recovery
The easiest and most common method is a multiple charge with a small current with interruptions between charging. By the end of the first and subsequent charges, the voltage on the battery rises, and it ceases to perceive the charge. During the break, the electrode potentials on the surface and in the depth of the active mass of the plates are equalized, with a more dense electrolyte from the pores of the plates diffuses into the interelectrode space and reduces the voltage on the battery during breaks. In the process of cyclic charge, as the capacitance battery set, the electrolyte density increases.
When the density becomes normal for this type of battery, and the voltage on one section will reach 2.5-2.7 V, the charge is stopped.
Multiple charging modes:
Charging current 0.04-0.06 rated containers. The time of the first and subsequent charges is 6-8 hours. The break time between charges is 8-16 hours. The number of cycles (charging) is 4-6 hours.
J Zap. \u003d 0.04 + 0.06 * CN.
Restoring a lead battery, with not complete loss of tank.
To restore the battery, which lost the capacity - dissolve sulfates (disulfate), you just need to apply on it, high voltage, and long, keep it up. However, the increase in voltage also increases the intensity of gas division. Therefore, we need to take pauses to calm the battery.
We take the battery that has lost the container due to sulfate. We pour water into it if he swallowed, but not much, about so many cubic centimeters as the ampere hours passport. And then maybe less. Connect it through the relay, time to the current source, which connects the battery to the source for 13 minutes and disconnects for 13 minutes. First, we give 14.3-14.4 volts, we make full 2 \u200b\u200bcycles. Hold under the voltage, after it reaches the tuned value, on the battery, in this case, 14.3-14.4 volts, a day. After what increase the voltage to 14.5-14.6 V, we also make two cycles. After that, we increase the voltage up to 14.8 V, and we make as many cycles as long as the control discharge, do not detect a sharp reduction in the capacity of the tank. The cycles are needed, not only for tracking, as well as the capacity is added, but also so that the electrolyte is stirred, with repaired acid, from lead sulfate. After the battery was restored, then plunge the water, until we see that the water stopped getting absorbed, closely watch not to pour. After that, a pair of cycles to wash the electrolyte should be done, but you do not need to charge a large voltage.
Experimental data
For experiments with a disulfate process, a time relay was made, which, turned on the current supply, for 13 minutes and disconnected for 13 minutes. Conditions and time of voltage, roughly the same. Time actions, about a day.
If you feed, on a sulfatized battery 10 Ah voltage 14.3 volts, a day, 13 minutes, after 13 minutes. After that, we carry out a controlling discharge on a 2 amp light, then an increase in the luminescence time of this light bulb is 6-7 minutes if optically accumulator, such a container, it shines 5 hours. When filing 14.5 volts, for the same session, 10-13 minutes of the glow is added. When filing 14.8 volts, 24-29 minutes of capacity is added. In all cases, there is a strong gas empowering, the more voltage, the topics and the gas empowerment.
From this data it follows that it is more profitable for disulfation to serve 14.8 volts.
Adding the tank occurs at the time of the voltage, and depends on the time of operation.
Optimal time, I consider 1 day voltage action 14.8 volt. That is, after reached a 14.8 volt voltage, you need to hold the battery day, through the time relay, 13 minutes after 13 minutes.
Due to the fact that in disulfation there is a strong gas empowerment, I recommend that the water does not pour a lot, pour as many cubic centimeters as the amp-hours has a battery through a passport. In order for the pores, to exit gas, otherwise mechanical gas exposure, can squeeze the naval.
Restoring the capacity of batteries quickly, but not very simple
It is characterized by high efficiency and efficiency (the battery is restored in less than an hour).
The discharged battery is preloaded. The electrolyte is drained from the charged battery and washed 2-3 times with water. A flush battery is poured an ammonium solution of trilon B (ethylenediaminetetrauxuscase sodium) containing 2 weight percentage of trilone b and 5 percent of ammonia. Time desulfation with a solution - 40-60 min.
The desulfation process is accompanied by the release of gas and the occurrence on the surface of the solution of small splashes. Termination of gas division indicates the completion of the process. With severe sulfate, the solution to the solution should be repeated.
After processing, the battery is washed at least 2-3 times with distilled water, then filled with normal density electrolyte.
The flooded battery is charged with a charging current to the rated tank according to the guidelines.
On the issue of preparation of the solution, it is advisable to apply to enterprises with chemical laboratories. The solution is stored in a darkened place in a vessel with a hermetic lid to avoid evaporation of ammonia.
Restoration of the container by disulfation by constant, stabilized voltage.
This recovery method has 100 percentage efficiencyIn other words, if it is not possible to restore the battery in this way, it will not be possible to restore it in any other way. I restored all sorts of batteries and with a complete loss of tank, the voltage on which was zero volt (0.5V), and not fully loss when the voltage is less than 13.0V.
The method itself is very simple.
We supply 14.7 - 15 volts (limit the current up to 1.5 amps if the battery is 10-15 Ah) to the battery that has lost the capacitance, and so leave for 12-15 hours. The battery will boil, but not to be afraid, it should be.
After that, we discharge a bit, for example, we connect a light bulb so that the electrolyte mixed.

Further put on charging as well as the first time: we give 14,7-15 volts (the voltage will seek, but it should not exceed 14.7-15 volts, when the battery charges, that is, limit 14,7-15 c), and so We leave another 12-15 hours.
After that, we turn off the voltage stabilizer, and we give a battery somewhere in a day, after which we do the stress measurement, which should be around 13.0-13.2 volts at +20 degrees.
If the voltage is less than this magnitude, repeat the recovery cycles until the voltage is raised to the specified numbers.
If the voltage on the battery does not reach 13.0 V, and somewhere around 12.7 V, it may also be bad, for a weak electrolyte density it normal tension. If the voltage did not reach 10 volts, this battery is broken mechanically: the plates were closed, plates were attacked, etc. Such battery road only on scrap metal.
It is better, of course, to make a control discharge after each recovery cycle to have an idea of \u200b\u200badding or adding a container. To do this, we find a light bulb with such a load so that the battery is discharged for 4-5 hours, so that we do not wait a lot and measure the discharge time, but do not care, the battery voltage cannot be allowed below 10.5 V when discharge.
Another very important remark. If the battery is sealed AGM or gel, then do not leave the valves open, the air should not flow into the plate, otherwise the container will be lost. Before restoring such batteries, it is advisable to add water. To do this, we tear off the top plastic cover to get to the rubber valves, raise the valves and tighten the distilled water with the syringe, but not much so that the water is slightly covered with the plates (not to pour more!). To see the water, you need to shine something, for example, a flashlight lighter. We close the valves, apply the lid on top and soak tape.
If the battery lost all the capacity, this is when the voltage is less than 10 V.
We connect the recoverable battery to the stabilized voltage source on which the 15 V must be configured (the current is limited to 1/10 from the battery tank). And wait for the clock 15. At this time, I look at time from time to time, at some time the battery starts a slow taking current, and the voltage will fall at this moment, then the current will increase to the maximum and the voltage will fall to the lower point (usually about 12.4 c), after that time we are waiting for 15 hours so that the battery is charged. Then we restore the battery as partially lost the container (see above).
There are such cases when the battery does not start taking the current and after 15 hours. Then you should increase the voltage up to 20 volts, I added and more, sit a little a few minutes and look at the current, can go right away.
If the current did not immediately go, then you need to look more often, the main thing is not to skip the moment when the battery charges so that the voltage does not exceed 15 V, then we need to limit the voltage as quickly before charging.
Yes, there is still a very important remark, do not stop the recovery process on the floor path, be sure to complete the cycle.
Restoration of the battery by a short-term current pulse of a large value.
Sometimes it happens that as a result of any reasons, the plates of one of the batteries cans in some way they closed and the charge becomes impossible.
It is logical to assume that the cause of the closure can be eliminated by burning the problem area. To do this, the battery is connected to a very strong current source, at least 100 amps, for example, a welding machine, with a rectifier diode at the output. The chain is closed for 1-2 seconds, during this time the cause of the closure should evaporate due to strong overheating.
Several applications and the effectiveness of this method in practice.
Personally, I came across one 7 A.Ch. lead battery CSB with a closed can. The battery ran several years without charging. The reason for the closure was most likely that the battery plates due to abundantly laid sulfate were fed, and the separator stuck.
By connecting to the welding machine for 2-3 seconds, the closure was eliminated, but the subsequent recovery measures were unsuccessful, which is not surprising, because completely lost the tank lead unqualified batteriesnot restored. But the use of this method to other types of batteries can be quite reasonable.
Example 2.
About your experience Application of this method to Nickel Cadmium (NICD) accumulator, I told one friend, in such a way it was possible to reanimate and put into operation a mine nickel-cadmium battery, "KCSL 12", for the boon.
Example.
Another familiar daded a lithium-ionic (Li-Ion) battery from a portable player DVD. IN lithium-ion batteries With a deep discharge, a copper, closing the shunt between the plates, is sometimes formed. The result of the recovery was such that the battery capacity was higher than it was at the moment when he was new.
Recovery of serviced batteries in particular automotive.
There is one way able to restore your battery.
The essence of the method.
Pour the entire electrolyte. Pour distilled water into the battery to the level of plates coating. Connect to the battery constant voltage of about 14 volts and leave for 1-2 hours. After that listen to the battery, if you hear that it is boil, slightly reduce the tension. We leave for half an hour and listen again: our task is to keep such a battery voltage so that the gas evolution is minimal, but that it was.
Hold, under such voltage, battery for a week, and better two. After that, distilled water in the battery will turn into an electrolyte of weak density, due to the dissolution of lead sulfate and its transformation into a sulfuric kilot molecule as a result chemical reaction. We drain the entire electrolyte, and pour distilled water again. Also, connect the voltage, follow the battery a bit, sometimes let the bubbles, and keep 1-2 weeks.
If the electrolyte does not change the density anymore, then you can stop disulfate.
After that, we drain the resulting weak electrolyte and pour the electrolyte of normal density. We connect your charger and charge the battery as usual, to the state of full charge.
After that, it is necessary to measure the density of the electrolyte and align to normal density in all banks.
All your battery is restored.
If you have nothing to measure low density electrolyte, then, just in case, you can perform one more, third, such a cycle.
The specified procedures apply it makes sense if the battery plates are still integer if the precipitate is clearly sedimized in your battery especially with slices of lead plates, it is clearly not worth it.
Read 5 min. Views 114. Published November 26, 2015
In this article we will tell you how to restore the battery with your own hands.
Motorists with experience probably came across or his complete failure. In such situations, it is not necessary to throw the battery in the scrap and buy a new one, you can try to restore the battery. In this article we will tell you how to restore the battery with your own hands.
During the operation of the car there are many situations when the battery fails. The output battery has several reasons. We will consider them below. The main reasons for the output of the battery are found in the table below.
| Cause | Description |
| Age of automotive battery | Pretty old battery, whose age is rapidly approaching 10 years, it will not be possible to restore. Any restoration of such a battery does not help. |
| Lack of electrolyte | Due to the low quality or lack of electrolyte, the battery can also fail, and it will be necessary to restore it. With a lack of electrolyte, it is necessary to inspect the entire battery case. The electrolyte could simply lean through the crack. |
| Strong frosts on the street | At a pretty cold period of the year, the battery may not keep charge and quickly fail. Experienced motorist It knows that the strong temperature differences can quickly kill any reliable battery. |
| Circuit Rechargeable Battery Plates | If the plates are closed in one battery section, then the entire battery can go with you. It is possible to determine the closure of the plates by boiling electrolyte in one of the sections. |
| Damage to the carbon plates of the battery | Damage to coal plates can be determined by staining electrolyte into black. |
| Sulfating of the plates of the battery. | With sulfatization of the plates, the battery will also not keep charge and fail. |
Separately, it is worth mentioning the impact of low temperatures on the batteries of cars. Due to severe frosts in the battery, the sides can bloom, and then drop out the electrolyte as soon as you start charging the battery. All these signs are talking about the watery of the battery. If the battery has been pretty low temperatures And washed, then it will not be possible to restore it, as there will be many short circuits in different batteries.
Next, we will tell about the possibilities for restoring the car battery. Also they will be told the best ways Recovery of car batteries. Moreover, these methods are suitable for the restoration of acid batteries, as well as such batteries that were exploited by improper way.
 During the operation of the car there are many situations when the battery fails. The output battery has several reasons.
During the operation of the car there are many situations when the battery fails. The output battery has several reasons. Car battery recovery
An independent recovery of the car battery is impossible without using certain materials and tools. To restore the battery with your own hands, you will need the following tools and materials:
- Pipette,
small enema,
- concentrated electrolyte,
- distilled water,
- Charger in which you can adjust the current level,
- electrolyte density meter,
- Areometer,
- Additive sulfate for electrolyte.
The first way is suitable for the restoration of such batteries, the work of which occurred with a minimum charge, equal to almost zero. To restore such rechargeable batteries for the car, it is necessary to use for them a long-time charging cycle.
The long-term charging cycle procedure must be used for the tired batteries at least twice. This reduction method is also suitable for a battery with plate sulfate.
The second way to restore batteries implies a complete resuscitation of the battery. This method can be applied when restoring acid battery. To do this, it is necessary to pour out the entire electrolyte from the battery sections, then rinse its insides with distilled water.
 We will write a couple of ways to restore the battery with your own hands.
We will write a couple of ways to restore the battery with your own hands. For flushing batteries, only distilled water is suitable, otherwise, on the inner walls of the battery, third-party impurities and salts are mounted, which are present in conventional tap water.
After flushing the battery, we dilute the electrolyte with distilled water or a special additive. After that, we pour it back to the battery and connect it to the charger. With the first charging after washing the battery, it is impossible to close the lid through which the electrolyte-water mixture was poured. The fact is that the battery can highlight gas at the first charging, and if the inner part of the battery is closed, then the gas accumulation can lead to an explosion due to excessive pressure inside. By typing the first complete charge, the battery must be discharged using any electrical appliance connected to it. As soon as it is completely discharged, it is necessary to charge again until the complete charge.
The discharge and charging cycles must be carried out when rechargeable battery as long as the voltmeter shows us the voltage at minimum of 14 V. The battery capacity can be calculated using the battery discharge to 10.5 V and subsequent charging. At this point, you will need to check the charging time and the charging current. These two indicators will need to multiply to obtain the battery capacity.
Basic Rules of Acid Acid Battery
In order for your battery to serve as long as possible, you need to stick to:
- Protect the battery with low temperatures in winter. If the weather is predicted for the night with a sharp decrease in temperature, then you can not leave the battery inside the car, you must pick it up with you warm room.
- Controlling the level of electrolyte in battery sections. It is necessary to regularly check the electrolyte level in all battery sections. If the level is insufficient, the electrolyte must be replenished with distilled water.
- Control the battery power compliance and charger. Using an excessively powerful charger will affect the durability of the battery.