There are several reasons why the battery capacity prematurely begins to fall. The main is the sulfate of the plates, which grows from frequent submissions, deep discharges or long-stored discharged batteries. Sometimes restored batteries, especially from the budget segment, can serve longer than only purchased. From this article, you will learn the reasons for the degradation of batteries, which will allow you to continue to properly exploit them, significantly extended life.
Why do accumulators degrade?
Each cycle of charge-discharge of the battery causes various kinds of structural damage to the plates. As a result, the battery capacity gradually decreases and every time it matters more and more, and it is discharged faster.

How does such a degradation process occurs? A detailed study under the electron microscope has revealed that the batteries are degraded by the principle similar to the propagation of corrosion in the metal. More specifically, it begins at different points of the perimeter of the plates, and then grow up on the entire surface. If it was possible to obtain an accurate map of the spread of erosion on the material, then it would be possible to develop methods to combat this problem, which would further contribute to the improvement of the performance of batteries.
Studies have shown that the battery is discharged significantly faster when operating at high voltage. For example, at 4.3, the battery degrades slower than at 4.7 V. This problem is further seen in more detail by studying chemical reactionsoccurring in batteries.
Acid batteries restoration methods
Let's start with the most serious malfunctions and methods of combating them. The rechargeable battery with the problem of sipping and closing the plates is completely contraindicated, as this not only will not give positive results, but, on the contrary, will speed up the process. First you need to drain the electrolyte, rinse with the containers with distilled water until all the dirt is washed. Carefully spend the procedure and do not be afraid to turn the battery.If the plates crumbled very strongly, which indicates a huge amount of garbage, then it is not worth loading ourselves with useless work. The battery is time to rest. But if everything is not so bad, then continue. It often happens that, by removing the discarded particles, it is possible to get rid of the short circuit.
Next, it is necessary to carry out desulfate plates - removal of salt sediments. There are two ways to hold it. The first is to purchase a special desulfatoric additive to the electrolyte. The second is using a special charger. If you have chosen the second option, then specify when you purchase whether such a mode is provided in the device. So, we turn to a detailed consideration of the technology of restoration of the automotive acid battery.
 1.
Take a pure electrolyte with a density of 1.28 g / cm3 and dissolve the additive in it for desulfation. It will take two days. Regarding proportions and other nuances, you can all read in the instructions.
1.
Take a pure electrolyte with a density of 1.28 g / cm3 and dissolve the additive in it for desulfation. It will take two days. Regarding proportions and other nuances, you can all read in the instructions.
2. Pour the electrolyte in the battery and check the density, it must correspond to the above nominal.
3. Remove the battery cans jams and connect the charger. Next, spend several charge-discharge cycles so that the battery capacity comes to normal. It is necessary to charge a small current, about 10% of the maximum allowable. Make sure that at this time the battery does not burn and did not boil. When the voltage on the battery terminals stabilized in the range of 13.8-14.4 V, reduce the current to 5%. If after a couple of hours, the electrolyte density has not changed, it means that the battery charged, you can end the process.
4. Now the time comes to adjust the electrolyte. If it is not nominal density, you need to bring up to 1.28 g / cm3, topping distilled water (if the density is higher than necessary) or a more dense electrolyte (if the density is below).
5. Next stage - Discharge. Connect the load and limit the current up to 1 A, and 0.5 A for a 6-volt battery. Wait until the voltage on the terminals is dropped to 10.2 V, for a 6-volt battery - 5.1 V. Section the time, since this parameter is important for measuring the battery container. It is calculated by the discharge current, multiplied by the time of this discharge. If it is below the norm, repeat the cycle until the container does not get the nominal value.
 6.
The process approached the end. Add some more additives to banks and tighten the plugs. Congratulations, such a battery will serve for a few more years. There is a faster way to restore automotive batteries. It will take about an hour. You need to do the following:
6.
The process approached the end. Add some more additives to banks and tighten the plugs. Congratulations, such a battery will serve for a few more years. There is a faster way to restore automotive batteries. It will take about an hour. You need to do the following:
1. Charge the battery to the maximum.
2. Drain the electrolyte.
3. Rinse several times with distilled water.
4. Fill a special trillion solution B with a content of 2% trillion b and 5% ammonia.
5. Wait for about 40-60 minutes. It will be seen how the reaction occurs. If the case is severe, the procedure will need to repeat several times.
6. Drain the solution and rinse again three distilled water.
7. Fill a new electrolyte and charge the battery with a rated current.
- To serve the battery longer, check the level and density of the electrolyte solution times. It, as a rule, rolls out from reloading or in hot time, therefore, the density increases. Tighten the distilled water, bringing it to the nominal.
IN winter time Raise the density slightly higher than the nominal, up to 1.40 g / cm3, just no more.
Calculate the battery with a rated current - 0.1 of its capacity in amps-clock. For example, if its capacity is 55 a / h, then charge it with a current of 5.5 A.
 - Do not leave the battery in winter in the garage that does not heal. It can climb and become unsuitable for use. Strong frosts are not every battery withstand, especially if it is old or fully discharged.
- Do not leave the battery in winter in the garage that does not heal. It can climb and become unsuitable for use. Strong frosts are not every battery withstand, especially if it is old or fully discharged.
Support the battery in a clean state to protect from current leakage and other unexpected troubles. This will increase its operational period.
Replacing electrolyte
Visit the nearest service center and lay out some amount of money, or replace the electrolyte yourself - your business. But it will be more pleasant, of course, do it with your own hands. On the preparatory Stage You will need the following:
Tara, where you will merge the old electrolyte.
Rubber pear for suction of electrolyte residues.
Charging and starting device with voltage in 12 V.
Aerometer that will measure the density of electrolyte.
Plastic or porcelain leak (can be used homemade).
Long rubber gloves with high protection.
Nominal density electrolyte solution.
Go directly to the procedure itself:
1. Disconnect the battery from the terminals and put on the smooth plane.
2. Remove the protection and unscrew the covers.
3. Rubber pear remove the old electrolyte.
4. If the electrolyte hit the open portions of the body, rinse them immediately with soapy.
 5.
Rinse the contents of jams by distilled water to the complete removal of the old sulfur solution.
5.
Rinse the contents of jams by distilled water to the complete removal of the old sulfur solution.
6. Wipe your pure wind dry.
7. Open the new bottle with a solution and filter to the level of plastic chips.
8. Measure the density of the electrolyte with a steeometer, it must be nominal - 1.28 g / cm3.
9. Connect the battery to the charger, and so, by the "Charging-discharge" cycle, until the density is completely restored. The strength of the current should be no more than 0.1a.
What to do if the rechargeable battery is unbearable? Everything is simple enough. Perform the same actions in order that are previously described, only you need to delete item 2. At this stage, take a drill with a drill 12 or 14 and over each bank drill holes. There is simply no other way, but you still need to merge the old electrolyte. After paragraph 9, cut out small plastic circles a little more than the diameter of the holes done and decompose evenly above them. Gas burner melt plastics so that it seals the capacity as thick as possible, in order not to spill the sulfuric acid composition. It can destroy the plates, which will lead to the complete unsuitability of the battery.
Greetings to you friends. Today I will tell you about the very effective method Recovery of tank in lead acid batteries.
In the period even the most properly operation, battery every day loses its container. And at one fine moment of his charge lacks to start the car engine. This example is exacerbated with the arrival of cold weather.
Naturally, the car enthusiast puts the battery for charging and after some time sees that the battery does not charge, and the voltage during charging stands as normal - 14.4-14.7 V or higher (12.6 without a charger).

Then if there is a loading plug, the check is made to it and it turns out that under the load, the voltage is very sensitive. Everything indicates the battery loss. The reason for this is the sulfate of the plates.

Usually, with proper operation, this occurs in about 5 years. This is a very good indicator. And here there is a way out - buy new battery. But, if you want to save money (as the batteries are not cheap now, and extend the battery life for another couple of years, then it is necessary to carry out its service. And not a simple, but a special that can reanimate the battery.
What batteries can be restored?
This method is suitable for batteries, which during the period of operation have not been susceptible to serious current or mechanical damage. And they were unusable as a result of temporary, natural sulfate.This method is not suitable for rechargeable batteries in which there is an inner swap plate, there is an internal closure of cans, there is a swift or other mechanical damage.
The method is excellent for desulfation of plates and is called the people by the method of "revroves" of the battery.
I divide the recovery battery into three stages.
Battery recovery process
Stage First: Preparation
The first thing is not necessary, but you need to do this to clean the surface of the battery from any contamination. Rinse with detergent all surface.Further, to be visually verified in the absence of damage on the housing, in the absence of fuses and convexities on the sides.
Second, open all jacks of cans and make sure that the electrolyte is available. If it is not in one of the cans, then you need to make sure there are no cracks on the case.
Then, with the help of a flashlight, inspect the plates inside - ships should not be. Here just for one one can clearly see sulfate - white bloom on the plates.

If everything is in order - add distilled water to each jar to the level. It will not be superfluous to measure the density of the electrolyte of each compartment.
Stage Second: Classic Recovery Method
Before switching to the storage of the battery, you must test the usual restoration method that has already become classic.Step one: We charge the battery to a complete charge of 14.4 V.

Step second: The halogen light bulb or another load is discharged by the battery to 10.6 V (the voltage is measured under the same load).

We repeat the cycle of these two steps 3 times and charge the battery for complete. Checking the container load fork or starter in the machine work. If the battery recovered - well - continue operation. If not, or not enough, then proceed to the third stage.
Stage Three: Rechacter Revolutionary Battery
This battery recovery method is the most effective of all existing ones. And reanimits the battery in almost 90% of cases.Step one: We hang on the battery load in the form of a halogen lamp, and discharge the battery at zero. The lamp will go through after a day (it all depends on the initial capacity of the battery). We leave the battery with the connected lamp for another 2-3 days to finally discharge the remnants.
Step second: Charging the battery reverse current. Connect the charger on the contrary: plus to minus, and minus to the plus. In order not to spoil your charger (or so that the protection against short circuit does not work), the battery consistently connect the same halogen lamp. And charge the battery in reverse polarity. After the voltage rose to Volt 5-6, the chain lamp can be excluded. Charge current It is advisable to put 5 percent of the battery capacity. That is, if the capacity of 60 amps-hours, then the charge current in the opposite direction is 3 amps. At this time, all the banks with electrolyte begin to be actively brown and hiss it is normal, since there is a reverse process.

I charge about a day, until the voltage appears 12-14 V. In the end, you turned out a fully charged battery with which at the output of the plus - minus, and on the minus - plus.

Step Three: Again, completely discharge the battery with a halogen lamp for a couple of days. Then we produce the correct charge plus to the plus, minus to minus. We charge to full up to 14.4 V.
On this all actions are completed.
Recovery Battery Recovery Result
Typically, the result helps to increase the battery capacity to 70-100% of the factory, of course there are exceptions.Specifically, in my case, it was possible to raise the container by 95% - which is an excellent result. The plates disappeared white sulfate flying, and they purchased a black color like a new battery. The electrolyte has become more transparent and clean.
Battery recovery video
I recommend you to watch the video where the battery is completely restored, which is about 10 years old.Initially, there is a "routing" with a change in the polarity of food, and almost at the very end already full cycle Revolution.
Purpose of the battery - run the engine starter and maintain consumers in electrical on-board network, together with the generator. If the car battery does not perform functions, the restoration of working capacity or replacement is required. Knowing the principle of operation of the battery, the design, you can try to return the battery with your own hands.
Not always the first sign of the malfunction of the battery is the loss of tension. It can be detected, the body of the device has given a crack, or the terminals are covered with a salt tap. Restoring the housing integrity and cleaning the battery terminals with their own hands belong to the elimination of external breakdowns.
Internal faults of a car vehicle require recovery:
- capacities deeply discharged battery;
- chulfate sediment sediment in cathodes;
- the closure between the variant plates leading to the discharge of the electrolyte and warming the cans;
- sowing of the active mass with plates leading to a closure.
Restoration car battery It is impossible if it is possible if the deformation of the hull and plates occurred due to deep frozening. If the lead plates are destroyed, the case of the housing, the battery is disposed of.

Restoring the car battery do it yourself
If there is an almost new car battery, the owners are trying to perform the restoration of their own hands, the owners are discharged or due to a faulty generator. This is possible, but more problems in repair non-servant battery.
Regardless, whatever the operation is carried out, you need to remember protection. The electrolyte is a concentrated solution of sulfuric acid, well reacts with the skin, charring the skin. When cleaning the terminals, it is necessary to use rubber gloves, all levels of level and density of electrolyte in open banks lead in protective glasses.
Is there suspicions of microcracks in the case? Moisten the surface and put a litmus paper. If it blooms - look for leakage. But dust during cleaning terminals is also soluble and gives an acidic reaction. Consider it.
Any hull wash, electrolyte drain can be carried out in enameled or plastic dishes. Remember, when diluted with water, the temperature of the solution rises. Neutralize spilled electrolyte can be drinking soda.
We offer to see ways to restore the car battery do it yourself on video.
Restoration of the car battery after deep discharge
The battery does not produce electric energy, and maintains it converted to chemical. Voltage is the potential difference between the two terminals of the element. It must be equal to 2.1 V with full charge. During charging, positive particles are collected on the anode, absorbing electrical energy. Distinguishing, ions from the anode go to the cathode, give energy in the form of a pulse to the network of the consumer.
The conductor serves an electrolyte - a solution of sulfuric acid of standard density. During the discharge period, small PBSO4 crystals appear on the surface of the plates. But deep discharge leads to the formation of large insoluble crystals. This means the electrolyte is impoverished, becomes weaker and is not able to create the desired energy capacity. Education on the plates of insoluble sediment makes it difficult to pass the current, resistance increases. Battery sat down. The recovery of the battery charge depends on the destruction of zinc sulfate sediment.

Another reason for the loss of the capacity may be short circuit in one or more elements. Negative and positive plates are separated by separators. But the blow, permanent shaking, a bad fastening of the housing in the nest can cause displacement of the plates, their contact. The sign will be the heating of the case, the loss of general voltage by 2.1 V (non-working bank). Restoration of the capacity of the AKB with CW requires replacing the cans or exposure to a pulsed current in 100 A.
Recovery of the capacity of the automotive battery
Even if there was no deep discharge, but the battery operates in a dilapidated state, the plate sulfate will occur inevitably. The thicker precipitate, the lower the electrolyte concentration, the battery capacity.
The capacitance schemes of the capacity of the battery are to restore the density of the electrolyte and the ability of the battery to receive a charge.
- Disassembly of plates and their mechanical cleaning is used if another method is only disposal. The holes are cut into the housing cover, remove plates. Distilled water was washed cavities and plates. The tightness of the design is restored, the electrolyte is poured, charging is performed. But since the plates are fragile, the restoration of the acb in this way - the job is jewelry.
- Chemical dissolution of crystals can save the fully sex battery. The active substance is a trildon solution B. should be discharged by the battery, drain the electrolyte, rinse the insides with distilled water. In pure banks, pour a 2% trilon solution b and 5% ammonia in a calculation for the entire volume. Within an hour, boiling and gas formation will be observed. Perhaps the solution will have to fill it repeatedly if the reaction of the dissolution of the sediment continues. After draining the solution, rinse with distilled water and pour fresh electrolyte. Carry charging.

Charger for restoring car batteries
- The dissolution of crystals at an early stage by the method of a control cycle. You will need, ammeter and voltmeter, consumer of energy. The principle of recovery of the car battery density is to use multiple charging cycles with full battery discharge. The operation is performed with your own hands but requires a lot of time.
Charging is conducted with a current of 0.1 from the initial battery capacity. The density of the electrolyte is measured in each bank, is communicated to the norm, for mixing the charging is still half an hour. After the incandescent lamp is connected to 70 V as the current consumer. At a voltage of 10.2, the battery is considered discharged. The discharge time determines the remaining battery capacity. The new battery is discharged 10 hours.
The cycle is repeated several times, sulphate crystals dissolve, the resistance drops, the battery discharge time increases. The process of cleaning the plates from the sediment must be continuous. it the best way Restore the old or maintenanceable car battery.

- You can dissolve sulfate stone without chemistry, using only distilled water. Akb's flooded water is charged, under the voltage of 14 V. Weak boiling in banks is maintained by voltage regulation. In the process, the density of the fluid varies - the dissolution of the precipitate is underway. Water changes several times, the process may take a month. After cleaning the plates with dissolution, the cavity is washed and poured with an electrolyte of the desired density.
- When no methods help the restoration of the car battery, use the surveillance. The method will help if the battery is high-quality, the electrolyte is transparent, just visible on the plates. Sulfates are deposited on the anodes. If the plate is minus, the precipitate will collapse. With a fully discharged battery, we connect to the reverse current by force of 6 A, we decrease to 2a, add resistance to reduce the acb bars warming up. Recovery is accompanied by boiling cans. After it is necessary to reassure the device. Capacity will return or the battery will finally collapse.
- There is a special charger for automotive batteries with a function of pulse mode and desulfation. Capacity recovery scheme:
charging on a small current 10 minutes;
discharge under load 1 minute.
The cost of the cost is commensurate with the price good battery. It is more often used to restore and charge automotive batteries. An ordinary charger.

If the bank closed in the battery
The first sign of the failure of banks will be the voltage drop to 10.5 V. The second is the boiling of the battery and the sulfate of the plates. Detect a faulty element by electrolyte density.
You can free up the jar from the electrolyte, rinse and remove the plate from it. After inspecting and eliminating damage, the contour is restored, searched. Sometimes the bank is replaced by a similar, from the non-working battery. The element is placed in place, the connection is restored from the car battery terminal.
The closed battery bank is the cause of its disposal. Sometimes a risky effect of exposure to the problem area by a pulse with a current of 100 A for 1-2 seconds is used. The location of the plates must melt - point contact and large resistance. However, it is worth risking if the battery is preparing to write off.
Video
We offer to watch a lesson how to restore a very old battery.
If your battery does not hold charge, stopped twist the starter - do not hurry to throw it out, in most cases it can be restored and it will serve a few more seasons. And if the battery is imported, then it can also survive a new one, from cheap of course. You can, because of the wrong operation and storage with it something happened, we will analyze the main fault of the batteries and the ways to repair them.
The most common cause of malfunction of old batteries is the proofatration of plates. In this case, the battery capacity drops significantly, sometimes almost to zero and naturally the battery power is not enough to twist the starter.
Some car enthusiasts immediately accuse the starter, but for the starter you need a good starting current, 100 and more amp. And if it is not, then I'm sorry - the starter is not here. If you do not have an instrument for checking the battery under load - take in advance competitive battery And try to get out of it.
The second reason is the destruction of coal plates, spindle plates. Such a battery can be restored in some cases, but not always. There is a sign of malfunction - dark, almost black electrolyte during charging.
The third is the closure of plates in some section. Detect this malfunction is also not a problem, the section is heated and the electrolyte in the section, as a rule, turns away. Restoring the battery with such a fault is more complicated, sometimes you have to change the plates in this section, but still cheaper than buying a new one.
The following fault refers to the discharge of improper operation and storage of the battery. It is known that discharged, or half a discharged battery on a strong frost can freeze. And the trouble is that during freezing there is damage to both the plates themselves and the battery housing.
As a result, the numerous closures between the plates, and when charging, the electrolyte boils very quickly. This battery is no longer possible. Therefore, caring auto-owners in the winter remove the battery and store somewhere in the warm room.
Now, with regard to the recovery of the battery. Let's start with more serious faults - swallowing and closing plates. You should not charge such a battery, it will not give anything, but rather the opposite. First, it is necessary to make a flushing with distilled water, until all the dirt is repeated from there. Do not be afraid to turn the battery. If the garbage is a lot, the plates trembled very much - most likely he is hopeless. Often, removing the shredded particles, the short circuit disappears.
So, the technology of recovery acid, lead batteries:
1. We take a fresh electrolyte (density of 1.28 g / cc.) Dissolve the desulphous additive in it (the additive is necessary to dissolve, 2 days). All nuances on the additive, how much should it, based on the volume of the battery, read the instructions.
2. Fill in the battery electrolyte, check the density with the range, it must be a nominal 1.28 g / cc.
3. Unscrew the plugs and connect the charger. Now we need to make several cycles charging-discharge to restore the battery capacity. We will charge a small current, about 1/10 part of the maximum. The battery itself should not warm and boil.
When the voltage is reached on the terminals of the accumulator 13.8-14.4 V, the charge current is still reduced by 2 times and measure the electrolyte density. If after 2 hours the density did not change - you can read it charged, and turn off the charging.
4. Now we make the electrolyte adjustment. I bring the density to 1.28 g / cc. Cm., I.e. nominal, topping distilled water or an electrolyte of high density (1.40 g / cc. cm.).
5. The next step is discharge. We connect the load (resistor or light bulb), and limit the current about up to 1a, and 0,5a for a 6 volt battery, we wait until the voltage on the terminals does not fall to 10.2V, for a 6-volt battery - 5,1V. We interfere with the time from the moment the load is connected. it important parameter To measure the battery capacity. The discharge current is multiplied by the discharge - we get the capacity of our battery. If it is lower than the nominal, then we repeat the discharge cycle until the battery capacity approaches the nominal.
6. All, the battery recovery process is finished, add to the electrolyte some more desulfating additives and twist the corks. Such a battery is capable of listening for more than one year.
There is another way to restore car batteries, faster, within 1 hour. It consists in the following:
The battery can be charged, then the old electrolyte is drained and is washed with distilled water 2-3 times. Then poured a special solution containing 2 weight percentage of trilon b and 5 percent of ammonia. We are waiting, the desulfation time is 40-60 minutes, and it can be seen as the reaction occurs.
In some cases, the procedure for desulfation must be repeated. Upon completion, we drain the solution and rinse 2-3 times with distilled water. Next, fill the electrolyte, charge the battery with a rated current ...
And finally some tips on proper care Behind the battery.
In order for the battery for a long time - check regularly, once a few months, the level of electrolyte and its density. The electrolyte turns away, as a rule, from reloading, or in the summer in the heat, then it is necessary to top up distilled water.
In winter, in the frost, if there is a need to ride, pick up the density of the electrolyte to 1.40 g / cc. Cc. But not more!
Charge your battery with a rated current - 0.1 from its capacity in amps clock, i.e. If its capacity is 55a / h, then charge it with a current of 5.5 amps.
Do not leave the battery for the winter in a not heated garage. It can freeze and come into disrepair. Frosts in -20-25 degrees not every battery can withstand, especially if it is discharged.
april 27, 2017
Like any product, acidic lead battery It has its own shelf life and with proper operation will last for quite a long time. Filed out of the power supply of the car is replaced with new ones, but in some cases it is possible to repair, after which the battery will last for some time. It should be known that the restored car battery will serve for some time, but by and large should be prepared for the acquisition of a new one.
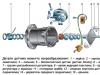
To better assimilate the information about which it will go below, we offer the reader to familiarize yourself with the automotive battery device. It is clearly shown in this scheme:

The main causes of the malfunction of automotive batteries
The most common malfunction of automotive batteries is. In this case, the battery capacity drops noticeably, and as a result, the device lacks the power to turn the starter.
It is possible to determine the sulfate of the plates by the following features:
- Reducing capacity;
- Electrolyte boiling;
- Overheating plates;
- Increased voltage on the electrodes.
The next reason for the faulty work of the AKB - destruction and Sleeping of Coal Plates. You can define this malfunction on dark electrolyte color. The restoration of the car battery in this case is possible, although not always.
The third common fault is associated with amykania lead plates in one of the sections of the battery. Reveal this breakdown is easy enough. When charging, the faulty section will be overly basked, and the electrolyte - pour out. You can restore the battery in this case, although it is somewhat more complicated than in the first case. The solution to the problem is to replace the lead plates in the section, which is quite consistent, although cheaper purchases of a new battery.
The fourth cause of the defective battery operation is connected with incorrect operation and storage of AKB. It is known that the non-fully charged battery at a minus temperature can freeze. As a result of freezing, lead plates may be damaged, as well as the device housing. This can lead to short circuits in the device housing and electrolyte boiling. In this case, unfortunately, restore the battery will not work.
Restoring the car battery do it yourself
 Finding out the reasons, you can proceed to the consideration of the methods of recovering the battery.
Finding out the reasons, you can proceed to the consideration of the methods of recovering the battery.
Elimination of sulfation
Sulfate plates leads to the fact that the charged battery does not produce full powerand the discharge occurs very quickly. To carry out the battery restoration work:
- Charger;
- Electrolyte;
- Distilled water;
- Protective glasses and gloves;
- Desulfating additive;
- "Ariometer".
The battery is fully charged, after which the electrolyte merges with it and it is washed. A new electrolyte is poured into the banks and the corresponding desulfating additive is added.
The rules of its use should be studied before the start of work. An electrolyte with a additive should be fully fill in the bank to a level recommended by the manufacturer. Within two days, the battery must draw, during this time the additive must eliminate deposits on the plates.
Recovery of capacity After deleting sediments, the power supply capacity should be properly restored. For this, charging should be carried out with small currents, not higher than the values \u200b\u200bof 0.1a. The battery is fully charged, check the density and, if necessary, align to the required values. Next, we carry out the battery discharge to a voltage of 10.5 volts, while the voltage in each bank should not be below 1.7 volts.
After deleting sediments, the power supply capacity should be properly restored. For this, charging should be carried out with small currents, not higher than the values \u200b\u200bof 0.1a. The battery is fully charged, check the density and, if necessary, align to the required values. Next, we carry out the battery discharge to a voltage of 10.5 volts, while the voltage in each bank should not be below 1.7 volts.
Battery capacity can be determined by calculating battery discharge time. To do this, multiply the charge current indicator for a while. If the battery capacity is lower than the nominal, charging-discharge cycles should be carried out until the car battery will occur.
Charging the battery capacity of the load You can use the car lamps sequentially connected. After that, the battery is fully charged, while the charging current should not exceed half of the usual indicators when charging. It is necessary to determine the power of the lamp and the discharge time to the specified quantities. According to the simple formula, the battery capacity is calculated and in the case of an insufficient capacity of the power supply cycle, the category - charge should be made before the acceptable values \u200b\u200bof the battery capacity should be achieved. After performing work in the electrolyte, you can add a small amount of additive to complete the plugs and use the recovered battery.
Deep sulfation
There are still methods, how to restore the car battery, which is almost completely overwhelmed. However, these ways are sufficiently dangerous and will require special premises for work.
Restoration of reverse current To perform the restoration, this method will require a power source of high power. For example, for this it will suit the welding transformer (not to be confused with the inverter). At this source, the output voltage should be at least 20 volts, and the current is more than 80 amps. It should be borne in mind that the battery should not have a short circuit of the plates, in this case the consequences can be unpredictable. Recovery is carried out by reverse current, for which the transformer plus connect to minus the battery, and minus to the plus terminal of the AKB.
To perform the restoration, this method will require a power source of high power. For example, for this it will suit the welding transformer (not to be confused with the inverter). At this source, the output voltage should be at least 20 volts, and the current is more than 80 amps. It should be borne in mind that the battery should not have a short circuit of the plates, in this case the consequences can be unpredictable. Recovery is carried out by reverse current, for which the transformer plus connect to minus the battery, and minus to the plus terminal of the AKB.
Charging the battery probe of the recoverable power supply must be turned out, and the electrolyte level correspond to the norm. Charging is included for 30 minutes, while it is formed abundant gas formation and plenty of heat is formed, the electrolyte can even be peeling from the Gorlowin. Therefore, security measures should be observed. At the end of the charge, the electrolyte is drained with reverse current, washed with distilled water and poured a new solution of sulfuric acid of the required density.
Next, charging is carried out ordinary charger Proper polarity minus to minus, plus to the plus. At the end of the charging, you can spend several control cycles. It should be remembered that these works do not guarantee recovery and can lead to the final battery output.
 This method, like the previous one, should be carried out on the battery, which, in case of failure, it will not be sorry to dispose. The battery is charged as much as possible, drain the electrolyte and washed with distilled water. The release capacity is poured with a solution of sodium ethylenediaminetetretetraux. For its preparation it is better to use the chemical laboratory.
This method, like the previous one, should be carried out on the battery, which, in case of failure, it will not be sorry to dispose. The battery is charged as much as possible, drain the electrolyte and washed with distilled water. The release capacity is poured with a solution of sodium ethylenediaminetetretetraux. For its preparation it is better to use the chemical laboratory.
The time that is necessary for the desulfation of the battery is from 40 to 60 minutes, while the gas isolate and the heating of the container occurs. At the end of the gas division, the solution is drained, washed 2-3 times with distilled water, poured a new electrolyte and battery charge. In case of good luck, the recovered battery will last for some time.
Correct operation of the car battery
And so that you do not have to wonder how to restore the car battery, it is worth taking a few useful Soviets about caring for this device.
- With a frequency once every two or three months, check the level and density of the electrolyte;
- In harsh frosts it is worth raising the electrolyte density to 1.40 g / cc. CM.
- Drop the battery must be current ten times less than its container. For example, if the capacity of the battery is 60 a / h, the charging must be performed by a current of 5 amp;
- At the air temperature below -25 'C should not leave the car overnight on the open parking lot. At such a temperature, the electrolyte in the battery can freeze, which will lead to the output of the battery.
When complying with these simple tips, you will be able to significantly extend the life of the AKB and do not have to be asked about how to restore the car battery.