ENZYMATIC REACTION KINETICS
studies the patterns of flow in time of enzymatic p-tions, as well as their mechanism; chapter chemical kinetics.
catalytic the cycle of conversion in-va S (substrate) into the product P under the action of the enzyme E proceeds with the formation of an intermediate. conn. X i:
where ki- rate constants of individual elementary stages,
formation of an enzyme-substrate complex X 1 (ES, Michaelis complex).
At a given t-re, the rate of the p-tion depends on the concentrations of the enzyme, substrate, and the composition of the medium. There are stationary, pre-stationary and relaxation kinetics of enzymatic p-tions.
Stationary kinetics. In a stationary state on intermediate Comm. (dX i/dt= 0, i = 1, ..., n) and with an excess of substrate , where [S] 0 and [E] 0 are the initial concentrations, respectively. substrate and enzyme, the kinetics of the process is characterized by a constant, time-invariant level of concentrations in between. comp., and the expression for the rate of the process v 0 , called initial stationary speed, has the form (Michaelis-Menten equation):
 (1)
(1)
where the values k cat and K m -> functions of the rate constants of elementary stages and are given by the equations:

The value of k cat
called efficient catalytic. process rate constant, parameter K m -> Michaelis constant. The value of k cat
determined by the quantities max. slow stages catalytic. districts and sometimes called. the number of revolutions of the enzyme (enzyme system); k cat
characterizes the number of catalytic. cycles performed by the enzyme system per unit of time. Naib. common, having a value of k cat. for specific. substrates in the range of 10 2 -10 3 s -1 . Typical values of the Michaelis constant lie in the range 10 -3 - 10 -4 M.
At high concentrations of the substrate, when that is, the rate of p-tion does not depend on the concentration of the substrate and reaches a constant value, called. Max. speed. Graphically, the Michaelis-Menten equation is a hyperbole. It can be linearized using the method of double reciprocals (the Lineweaver-Burk method), i.e., building the dependence of 1/v on 1/[S] 0 , or other methods. The linear form of equation (1) has the form:
 (2)
(2)
It allows you to determine graphically the values K m and v max (Fig. 1).

Rice. 1. Graph of the linear transformation of the Michaelis - Menten equation in double reciprocals (according to Lineweaver - Burke).
Value K m > numerically equal to the concentration of the substrate, at which the rate of p-tion is equal, therefore K m often serves as a measure of the affinity of the substrate and the enzyme, but this is only true if
Quantities K m > and change depending on the pH values. This is due to the ability of the groups of the enzyme molecule involved in catalysis to change their state of ionization and, thereby, their catalytic. efficiency. In the simplest case, a change in pH leads to the protonation or deprotonation of at least two ionizable groups of the enzyme involved in catalysis. If in this case only one form of the enzyme-substrate complex (for example, ESH) out of three possible (ES, ESH and ESH 2) is able to turn into a solution product, then the dependence of the rate on pH is described by the f-loy:

where f= 1 + / and f" = 1 +
+K" b/>-T. called pH-functions of Michaelis, and K a, K b and K" a, K" b -> group ionization constants a and b, respectively. free enzyme and enzyme-substrate complex. In lg coordinates - pH this dependence is shown in fig. 2, and the tangents of the slopes of the tangents to the ascending, pH-independent, and descending branches of the curve should be +1, 0, and -1, respectively. From such a graph, one can determine the values RK a groups involved in catalysis.

Rice. 2. Dependence of the catalytic constants from pH to logarithmic. coordinates.
The rate of enzymatic p-tion is not always subject to equation (1). One of the most common cases - participation in the district of allosteric. enzymes (see enzyme regulators) for to-rykh the dependence of the degree of saturation of the enzyme on [S] 0 is non-hyperbolic. character (Fig. 3). This phenomenon is due to the cooperativity of substrate binding, i.e., when the binding of a substrate to one of the sites of the enzyme macromolecule increases (positive cooperativity) or decreases (negative cooperativity) affinity for the substrate of another site.

Rice. H Dependence of the degree of saturation of the enzyme with the substrate on the concentration of the substrate with positive (I) and negative (II) cooperativity, as well as in its absence (III).
Prestationary kinetics. With rapid mixing of enzyme and substrate solutions in the time interval of 10 -6 -10 -1 s, transient processes can be observed that precede the formation of a stable stationary state. In this pre-stationary mode, when using a large excess of the substrate, the differential system. ur-tion, describing the kinetics of processes, is linear. The solution of this type of system of linear differentials. ur-tion is given by the sum of the exponential terms. So, for the kinetic scheme presented above, the kinetics of accumulation of the product has the form:

where A i ->, b and n -> functions of elementary rate constants; -roots of the corresponding characteristic. ur-tion.
The reciprocal of , called. characteristic process time:
For p-tion, flowing with the participation of nintermediate. Comm., you can get ncharacteristic. times.
The study of the kinetics of the enzymatic district in the pre-stationary mode allows you to get an idea of the detailed mechanism of catalytic. cycle and determine the rate constants of the elementary stages of the process.
Experimentally, the kinetics of the enzymatic solution in the pre-stationary mode is studied using the stopped jet method (see Fig. jet kinetic methods), allowing to mix the components of the district within 1 ms.
Relaxation kinetics. With a rapid perturbing effect on the system (changes in t-ry, pressure, electric fields), the time it takes for the system to achieve a new equilibrium or stationary state depends on the speed of the processes that determine the catalytic. enzymatic cycle.
The system of equations describing the kinetics of the process is linear if the displacement from the equilibrium position is small. The solution of the system leads to the dependences of the concentrations of the components decomp. stages of the process in the form of a sum of exponential terms, the exponents of which have the character of relaxation times. The result of the study is the spectrum of relaxation times corresponding to the number of intervals. Comm. involved in the process. The relaxation times depend on the rate constants of the elementary stages of the processes.
Relaxation methods kinetics make it possible to determine the rate constants of individual elementary stages of the transformation of intermediates. Methods for studying relaxation kinetics are different. resolution: ultrasound absorption - 10 -6 -10 -10
s, temperature jump - 1O -4 -10 -6 s, electric method. impulse - 10 -4 -10 -6 s, pressure jump - 10 -2 s. In the study of the kinetics of enzymatic p-tions, the application was found by the method of temperature jump.
Macrokinetics of enzymatic processes. Development of methods for obtaining heterogeneous catalysts by immobilization of enzymes on decomp. media (see Immobilized enzymes) necessitated the analysis of the kinetics of processes taking into account the mass transfer of the substrate. The regularities of the kinetics of p-tions were studied theoretically and experimentally, taking into account the effects of the diffusion layer and for systems with intradiffusion difficulties in the distribution of the enzyme inside the carrier.
Under conditions where the kinetics of the process is affected by the diffusion transfer of the substrate, catalytic. system efficiency decreases. The efficiency factor is equal to the ratio of the product flow density under the conditions of the flow of the enzymatic district with a diffusion-reduced substrate concentration to the flow, which could be realized in the absence of diffusion restrictions. In the purely diffusion region, when the process rate is determined by the mass transfer of the substrate, the efficiency factor for systems with external diffusion inhibition is inversely proportional to the diffusion modulus:

where
diffusion layer thickness, D - coefficient. substrate diffusion.
For systems with intradiffusion deceleration in first-order p-tions

where F T- dimensionless module (Thiele module).
When analyzing the kinetic regularities in fermentation reactors wide theoretical. and experiment. "ideal" models of reactors, a flow reactor (a flow reactor of ideal mixing), a flow reactor with ideal displacement, and a membrane reactor, have been developed.
Kinetics of polyenzymatic processes. In the body (cell), enzymes do not act in isolation, but catalyze the chains of transformation of molecules. R-tion in polyenzymatic systems with kinetic. points of view can be seen as consistent. processes, specific a feature of to-rykh is the enzymes of each of the stages:
where ,
resp. max, process speed and Michaelis constant i th stage of the district, respectively.
An important feature of the process is the possibility of the formation of a stable stationary state. The condition for its occurrence can be the inequality > v 0 , where v 0 is the rate of the limiting stage, characterized by the smallest rate constant and thus determining the rate of the entire sequence. process. In the stationary state, the concentration of metabolites after the limiting stage is less than the Michaelis constant of the corresponding enzyme.
Specific a group of polyenzymatic systems is made up of systems that carry out oxidizing.-restore. p-tion with the participation of protein electron carriers. Carriers form specific. structures, complexes with a deterministic electron transfer sequence. Kinetic the description of such systems is considered as an independent state variable of circuits with decomp. degree of population of electrons.
Application. F. r. widely used in research practice to study the mechanisms of action of enzymes and enzyme systems. Practically significant area of enzyme science is engineering enzymology, operates with the concepts of F. r. to. for optimization of biotechnol. processes.
Lit.: Poltorak O. M., Chukhrai E. S., Physical and chemical bases of enzymatic catalysis, M., 1971; Berezin IV, Martinek K, Fundamentals of physical chemistry of enzymatic catalysis, M., 1977; Varfolomeev S. D., Zaitsev S. V., Kinetic methods in biochemical research, M .. 1982. S. D. Varfolomeev.
Chemical encyclopedia. - M.: Soviet Encyclopedia. Ed. I. L. Knunyants. 1988 .
See what "ENZYMATIVE REACTION KINETICS" is in other dictionaries:
catalytic rtion cyclic. a process consisting of a number of elementary rations, the velocities of which are described by the acting mass law. This law has a simple form for ideal gas mixtures, ideal p moats, and ideal surface layers. ... ... Chemical Encyclopedia
Kinetics of chemical reactions, the doctrine of chemical processes, the laws of their flow in time, speeds and mechanisms. The most important areas of modern chemistry and chemical ... ... are associated with the study of the kinetics of chemical reactions. Great Soviet Encyclopedia
KINETICS CHEMICAL- (from the Greek. kinesis movement), a department of theoretical chemistry devoted to the study of the laws of chemistry. reactions. There are several types of chem. interactions and, above all, to distinguish reactions occurring in a homogeneous (homogeneous) medium from reactions that ... ... Big Medical Encyclopedia
- (biocatalysis), acceleration of biochemical. rations with the participation of protein macromolecules called enzymes (enzymes). F. to. a kind of catalysis, although the term fermentation (fermentation) has been known since ancient times, when there was no concept of chemical. catalysis. First… … Chemical Encyclopedia
- (from Latin re prefix, meaning reverse action, and actio action), the transformation of some in in (initial compounds) into others (products of the reaction) with the invariability of the nuclei of atoms (unlike nuclear reactions). Initial connections in R. x. sometimes called ... ... Chemical Encyclopedia
- (from lat. fermentum leaven) (enzymes), proteins that act as catalysts in living organisms. Main functions of F. to accelerate the transformation into into, entering the body and formed during metabolism (to renew cellular structures, to ensure it ... Chemical Encyclopedia
- (from the Greek pharmakon medicine and kinetikos setting in motion), studies kinetic. patterns of processes occurring with lek. cfd in the body. Main pharmacokinetic. processes: absorption, distribution, metabolism and excretion (excretion). ... ... Chemical Encyclopedia
At t=36-38 0 enzymes have the highest activity. This temperature is called the temperature optimum:
With an increase in t 0 to the optimum, the activity of enzymes increases.
High t causes enzyme denaturation.
Low t reduces the activity of enzymes.
A change in t 0 leads to disruption of bonds that fix the protein structure of enzymes (tertiary, quaternary), i.e. causes denaturation.
Reversible denaturation is observed with decreasing t 0 . This allows you to store enzymes, body fluids, blood.
An increase in temperature irreversibly disrupts the protein structure of the enzyme. This property is used in the sterilization of materials and instruments.
Fever is a protective property of the body, because. there is a denaturation of the enzymes of microorganisms and therefore it is inappropriate to use antipyretics.
The dependence of the reaction rate on pH
On the graph, this dependence looks like a bell. At the top of the curve there is a pH optimum point where the enzyme has the highest activity. pH affects the degree of ionization of acidic and basic groups. At different pH values, the active center can be in a partially ionized or non-ionized form, which affects the tertiary structure of the active center and the formation of the enzyme-substrate complex.
Effect of pH.
Enzymes, like all proteins, contain many positively and negatively infected groups (-NH 2 , -COOH), which are part of the amino acids arg, lys, asp, and glu. The total charge depends on the ratio between these groups. The charge of the protein-enzyme changes depending on the concentration of hydrogen ions in the cell, which neutralize (suppress the dissociation) of the carboxyl group:
and form positively charged groups:
Thus, an increase in the positive charge or a decrease in the negative charge on the surface of the enzyme is due to an increase in the concentration of hydrogen ions.
The state of a protein molecule in which the total charge of the protein is 0 is called the isoelectric state.
The pH value at which the charge of a protein molecule is 0 is called the isoelectric point (IEP).
Most enzymes are most active and stable around the isoelectric point.
Sharp fluctuations in pH promote protein denaturation, i.e. decrease in enzymatic activity.
The pH value at which the enzyme exhibits maximum activity is called the pH optimum, which is characteristic of a given enzyme reacting with a specific substrate.
Intracellular enzymes usually have an optimum pH corresponding to a neutral environment (pH = 7) close to the normal pH value for body fluids. There are enzymes whose optimum pH is in a strongly acidic and strongly alkaline environment.
Classification of enzymes.
There are six classes of enzymes:
1. Hydrolases - enzymes that break down the substrate with the participation of water molecules.
2. Lyases - enzymes that break down substrate molecules without the participation of water, while low molecular weight products are often formed - CO 2, NH 3, H 2 O.
3. Isomerases - enzymes that cause isomeric transformations in the molecule.
4. Ferases (transferases) - enzymes that transfer groups from one molecule to another or from one position to another within one molecule.
5. Oxidoreductases - enzymes that catalyze the transfer of protons and electrons (i.e. redox reactions).
6. Ligases (synthetases) - enzymes that catalyze the synthesis of large molecules from smaller ones.
Enzyme nomenclature.
The working name of an enzyme consists of the name of the substrate, the type of reaction catalyzed, and the ending -aza.
The systematic name consists of the name of the substrates, the name of the type of chemical transformation catalyzed, and the ending -aza.
The class name indicates the type of chemical reaction catalyzed by enzymes. Classes are divided into subclasses - specifies the action of the enzyme, as it indicates the nature of the chemical group of the substrate attacked by the enzyme. The subclass is divided into subclasses. Subsubclasses specify the action of the enzyme, specifying the nature of the attacked substrate bond or the nature of the acceptor.
I. Oxidoreductases catalyze redox reactions. Oxidoreductases are also called dehydrogenases or reductases. Oxidoreductases carry protons and electrons. Oxidoreductases are divided into subclasses:
1. Aerobic dehydrogenases - transfer protons and electrons to oxygen.
The coenzymes of oxidoreductases are:
NAD - nicotinamide adenine dinucleotide - contains vitamin B 5 - nicotinamide.
NADP - nicotinamide adenine dinucleotide phosphate, contains vitamin B 5.
FAD - flavin adenine dinucleotide, contains vitamin B 2 - riboflavin.
FMN - flavin mononucleotide, contains vitamin B 2 - riboflavin.
Oxidoreductases catalyze dehydrogenation reactions, i.e. elimination of hydrogen.
Oxidoreductases oxidize the following functional groups:
OH, -C \u003d O, -NH 2
Dehydrogenase coenzymes attach protons and electrons.
NAD-dependent dehydrogenases oxidize the following functional groups: alcohol hydroxyl (OH), aldehyde group (COH), amino group (NH 2).
NAD-dependent dehydrogenases catalyze the following types of reactions:
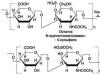
1. Dehydrogenation of hydroxyl groups
| lactate dehydrogenase |
COOH OVER + NADH + H + CH 3
lactate pyruvate
Lactic acid
2. Dehydrogenation of aldehyde groups (dehydrogenation of glyceraldehyde - 3 - phosphate)
| + OVER + + H 3 RO 4 | + NADH + H +
CH 2 OPO 3 H 2 CH 2 OPO 3 H 2
Glyceraldehyde-3-phosphate 1,3-biphosphoglyceric acid
3. Dehydrogenation of amino groups
UNSD UNSD
CH 2 + OVER CH 2
| | + NADH + H +
CH 2 glutamate dehydrogenase CH 2
Glutamic acid
FAD - dependent dehydrogenases oxidize (dehydrogenate) the following functional groups: the elimination of hydrogen from groups -CH 2 - CH 2 - with the formation of a double bond.
UNSD UNSD
| FADH FADN 2 |
CH 2 succinate dehydrogenase CH
UNSD UNSD
Succinate fumarate
2. Anaerobic dehydrogenases transfer protons and electrons not to oxygen, but to some other substrate. These enzymes are also called oxygenases.
II. Transferases are enzymes that catalyze the transfer of various groups from one substrate to another.
Transferase subclasses:
1. Aminotransferases carry out the transfer of an amino group from an amino acid to a keto acid. Catalyze the transamination reaction.
2. Methyltransferases catalyze the transfer of methyl groups (CH 3 -).
3. Phosphotransferases catalyze the transfer of a phosphoric acid residue. A subclass of phosphotransferases includes kinases that use ATP as a phosphate residue donor.
III. Lyases are enzymes that catalyze the rupture of C-O, C-C, C-N and other bonds, as well as reversible cleavage reactions of various groups, without the participation of water.
1. Carboxylase - addition of a carboxyl group (CO 2).
2. Dehydratase - the removal of a water molecule from the substrate.
3. Aldolases - cleave the C-C bond.
4. Hydratases - water enzymes on a double bond.
IV. Isomerases are enzymes that catalyze the transformation within a single molecule.
Catalyze isomerization reactions. Subclasses: mutases, tautomerases, racemases, epimerases, isomerases.
V. Hydralases are enzymes that catalyze the breaking of bonds in the presence of water.
VI. Ligases (synthetases) are enzymes that catalyze the union of two molecules using the energy of the ATP phosphate bond.
Influence of low-molecular substances on the activity of enzymes.
Low molecular weight substances that change the rate of enzymatic reactions are divided into 2 groups:
1. Activators - accelerating the course of an enzymatic reaction.
2. Inhibitors - slow down the course of enzymatic reactions.
Activators are divided into 2 groups:
1. Can act as an activator coenzymes or prosthetic group(mainly vitamins).
This group is characterized by the same patterns that are described for the interaction of the enzyme and the substrate F+S and A+Ko obey the same patterns
K m determines how much to enter Ko.
2. Activators that are a link between F and S (orientation of the enzyme and substrate) and ensure the interaction of the enzyme and substrate (F A S), the interaction of apoenzyme and the cofactor Apof A Ko
Often these are Me-Co, Mn, Mg, Zn ions.
Significance of enzyme activity inhibition.
1. Inhibition underlies the action of drugs and toxic agents.
2. Inhibition is one of the approaches to the study of the enzymatic action (for example, the structure of the active center).
Inhibition is of 2 types:
1. Irreversible
2. Reversible
Irreversible inhibition occurs when the binding of the inhibitor to the enzyme is irreversible.
For example: this is the action of alkylating agents (podacetamide) irreversibly acting on the thio group of enzymes. Irreversibility is due to the fact that the equilibrium is shifted to the right, towards the formation of a covalent derivative of the enzyme:
F-S-H + J-CH 2 CONH 2 F-S-CH 2 -CONH 2 + HJ
Irreversible is the action of toxic organophosphorus compounds, which are called nerve poisons, they inhibit acetylcholinesterase involved in the transmission of nerve impulses.
irreversible inhibition
Many inhibitors bind irreversibly to E or ES, and since this affects V max , this inhibition is considered non-competitive.
Inhibitors of this type often bind covalently to the enzyme or to the enzyme-substrate complex, irreversibly changing the native configuration. This explains the toxic effect of Hg 2+ , Pb 2+ and arsenic compounds.
The action of penicillin is based on irreversible inhibition. Penicillin inhibits the action of one of the enzymes involved in assembling the bacterial cell wall. Cells that do not have a cell wall are easily lysed.
The action of aspirin is based on the covalent modification of the enzyme. Aspirin reduces the rate of prostaglandin synthesis, acting as an inhibitor of the cyclooxygenase component of endoperoxide synthetase. It is believed that the occurrence of pain, inflammation, temperature is associated with prostaglandins.
In case of intoxication, the binding of poison or its displacement from the enzyme-inhibitor complex is possible with the help of reactivators, or antidotes. These include all SH-containing complexones (cysteine, dimercaptopropanol), citric acid.
Reversible inhibition is of 2 types:
1. Competitive
2. Non-competitive
Reversible competitive inhibition - the activity of the enzyme is restored after the removal of the inhibitor by increasing the concentration of the substrate.
A distinctive feature of a competitive inhibitor is that the competitive inhibitor is similar in structure to the substrate. A competitive inhibitor competes with the substrate for the active site of the enzyme.
Example: succinate dehydrogenase catalyses the conversion of succinate to fumarate. A competitive inhibitor of succinate dehydrogenase is malonic acid, which contains one CH2 group less than succinate.
COOH COOH COOH
CH 2 CH CH 2
CH 2 CH COOH
| | malonic acid
UNSD UNSD
Succinate and malonic acid are structural analogs and compete for the active site of the enzyme. (This is a confirmation that the active site is not a rigid formation, suitable for the substrate, like a "key-lock".)
With competitive inhibition, the degree of enzyme inhibition does not depend on the absolute concentration of the inhibitor, but on the ratio of inhibitor and substrate, if this ratio is J:S=1:50, then the activity of the enzyme is inhibited by 50%.
The action of a competitive inhibitor is removed by increasing the concentration of the substrate, since the affinity of the enzyme and the substrate is higher than the affinity of the enzyme and the inhibitor.
Km F and S and Km F and J are different and this is known by plotting Michaelis-Menten and Lineever-Burk
Vmax - the same
K m increases with the inhibitor.
The action of many chemotherapeutic agents is based on competitive inhibition. For example, sulfanilamide preparations used to treat diseases caused by microbial infections. Sulfanilamide preparations are structurally similar to p-aminobenzoic acid. PABA is a precursor in the microbiological synthesis of folic acid, from which the coenzyme is necessary for the synthesis of nucleic acids of microorganisms. With the introduction of sulfanilamide preparations, inhibition of the enzyme and the death of microorganisms are observed.
The use of fluorouracil, which is used in the treatment of cancer, is also based on competitive inhibition.
Noncompetitive, reversible inhibition.
The action of a non-competitive inhibitor cannot be eliminated by increasing the concentration of the substrate.
Non-competitive inhibitor not binds to the active site, it can bind to the free enzyme, or to the FS complex, or both, but both forms of JF and JFS are inactive.
K m - does not change, because no active site binding.
Vmax - decreases.
The most common type of non-competitive inhibition occurs under the action of reagents that reversibly bind the SH-groups of the cis located in the catalytic center or close to it. These are Cu 2+, Hg 2+, Ag + ions and their derivatives with the formation of mercaptides:
Enzymes that require Me ions for activation are inhibited in this way by agents that bind these ions:
ferro or ferrocyanide.
Regulation of enzyme activity.
The use of enzymes in pharmacy, medicine.
Types of regulation of enzyme activity:
1. Allosteric modification.
2. Activation of zymogens.
3. Regulation by chemical modification.
End of work -
This topic belongs to:
Structure, properties and functions of proteins
Elucidation of the structure of proteins is one of the main problems of modern biochemistry .. Protein molecules are high-molecular compounds .. Most proteins have a level of organization of the structure of the protein molecule ..
If you need additional material on this topic, or you did not find what you were looking for, we recommend using the search in our database of works:
What will we do with the received material:
If this material turned out to be useful for you, you can save it to your page on social networks:
COURSE WORK
Kinetics of enzymatic reactions
Introduction
The basis of the life of any organism is chemical processes. Almost all reactions in a living organism proceed with the participation of natural biocatalysts - enzymes.
Berzelius in 1835 for the first time suggested that the reactions of a living organism are carried out due to a new force, which he called "catalytic". He substantiated this idea mainly by an experimental observation: diastase from potatoes hydrolyses starch faster than sulfuric acid. As early as 1878, Kuhne called a substance that has catalytic power in a living organism an enzyme.
The kinetics of enzyme action is a branch of enzymatic study that studies the dependence of the rate of a reaction catalyzed by enzymes on the chemical nature and conditions of interaction of the substrate with the enzyme, as well as on environmental factors. In other words, the kinetics of enzymes makes it possible to understand the nature of the molecular mechanisms of action of factors affecting the rate of enzymatic catalysis. This section was formed at the intersection of such sciences as biochemistry, physics and mathematics. The earliest attempt to mathematically describe enzymatic reactions was made by Duclos in 1898.
In fact, this section on the study of enzymes is very important in our time, namely for practical medicine. It gives pharmacologists a tool to change the cell metabolism, a huge number of pharmaceuticals and various poisons - these are enzyme inhibitors.
The purpose of this work is to consider the question of the dependence of the reaction rate on various factors, how the reaction rate can be controlled and how it can be determined.
1. Michaelis-Menten kinetics
Preliminary experiments on the study of the kinetics of enzymatic reactions showed that the reaction rate, contrary to theoretical expectations, does not depend on the concentration of the enzyme (E) and substrate (S) in the same way as in the case of a conventional second-order reaction.
Brown and, independently of him, Henri were the first to put forward a hypothesis about the formation of an enzyme-substrate complex during the reaction. Then this assumption was confirmed by three experimental facts:
a) papain formed an insoluble compound with fibrin (Wurtz, 1880);
b) the invertase substrate sucrose could protect the enzyme from thermal denaturation (O'Sullivan and Thompson, 1890);
c) enzymes have been shown to be stereochemically specific catalysts (Fischer, 1898-1899).
![]()
They introduced the concept of maximum speed and showed that saturation curve(i.e., the dependence of the reaction rate on the concentration of the substrate) is an isosceles hyperbola. They proved that the maximum observed speed is one of the asymptotes to the curve, and the segment cut off on the x-axis (in the region of its negative values) by the second asymptote, i.e. constant in the rate equation, equal in absolute value to the substrate concentration required to achieve half of the maximum rate.
Michaelis and Menten suggested that the reaction rate is determined by the breakdown of the ES complex, i.e. constant k 2 . This is only possible under the condition that k 2 is the smallest of the rate constants. In this case, the equilibrium between the enzyme-substrate complex, the free enzyme and the substrate is established quickly compared to the rate of the reaction (rapid equilibrium).
The initial reaction rate can be expressed by the following formula:
v = k2
Since the dissociation constant of the enzyme-substrate complex is
K S \u003d [E] [S] / \u003d k -1 / k 1
then the concentration of the free enzyme can be expressed as
[E]=K S / [S]
The total concentration of the enzyme in the reaction mixture is determined by the formula
[E] t = [E] + [ES] = K S [ES] / [S] + [ES]
The reaction reaches its maximum rate when the substrate concentration is high enough so that all enzyme molecules are in the form of an ES complex (an infinitely large excess of substrate). The ratio of the initial speed to the theoretically possible maximum speed is equal to the ratio of [ES] to [E] t:
v / V max = / [E] t = / (K S / [S] + ) = 1 / (K S + [S] +1)

This is the classic equation Michaelis and Menten, which, since its publication in 1913, has been the fundamental principle of all enzyme kinetic studies for decades and, with some limitations, has remained so to this day.
It was later shown that the original Michaelis-Menten equation had several constraints. It is fair, i.e. correctly describes the kinetics of the reaction catalyzed by this enzyme only if all of the following restrictive conditions are met:
) a kinetically stable enzyme-substrate complex is formed;
) constant K S is the dissociation constant of the enzyme-substrate complex: this is true only if ;
) the substrate concentration does not change during the reaction, i.e. the concentration of the free substrate is equal to its initial concentration;
) the reaction product is rapidly cleaved off from the enzyme, i.e. no kinetically significant amount of the ES complex is formed;
) the second stage of the reaction is irreversible; more precisely, we take into account only the initial speed, when the back reaction (due to the actual lack of product) can still be neglected;
) only one substrate molecule binds to each active site of the enzyme;
) for all reactants, their concentrations can be used instead of activities.
The Michaelis-Menten equation serves as the starting point for any quantitative description of the action of enzymes. It should be emphasized that the kinetic behavior of most enzymes is much more complicated than it follows from the idealized scheme underlying the Michaelis-Menten equation. In deriving this equation, it is assumed that there is only one enzyme-substrate complex. Meanwhile, in reality, in most enzymatic reactions, at least two or three such complexes are formed, arising in a certain sequence.
Here, EZ denotes the complex corresponding to the true transition state, and EP denotes the complex between the enzyme and the reaction product. It can also be pointed out that in most enzymatic reactions more than one substrate is involved and two or more products are formed, respectively. In a reaction with two substrates, S 1 and S 2 , three enzyme-substrate complexes can be formed, namely ES 1 , ES 2 and ES 1 S 2 . If the reaction produces two products, P 1 and P 2 , then there may be at least three additional complexes EP 1 , EP 2 and EP 1 P 2 . In such reactions, there are many intermediate steps, each of which is characterized by its own rate constant. The kinetic analysis of enzymatic reactions involving two or more reactants is often extremely complex and requires the use of electronic computers. However, when analyzing the kinetics of all enzymatic reactions, the starting point is always the Michaelis-Menten equation discussed above.
1.1 The nature of the constantKin the equation
equation enzymatic reaction kinetics
The second postulate states that the constant K S in the equation is the dissociation constant of the enzyme-substrate complex.
Briggs and Haldane proved in 1925 that the original Michaelis-Menten equation is valid only for , i.e. when the equilibrium of the elementary stage E+S ES is established very quickly compared to the rate of the next stage. Therefore, such kinetic mechanisms (obeying the Michaelis-Menten initial condition and having one slow elementary stage, with respect to which equilibria in all other elementary stages are established quickly) are called satisfying the assumption of "fast equilibrium". If, however, k 2 is comparable in order of magnitude to k -1 ,
the change in the concentration of the enzyme-substrate complex over time can be expressed by the following differential equation:
d / dt \u003d k 1 [E] [S] - k -1 - k 2
Since we are considering the initial reaction rate, i.e. the moment when the reverse reaction does not yet occur, and the pre-stationary stage has already passed, then due to the excess of the substrate, the amount of the formed enzyme-substrate complex is equal to the amount of the decomposed ( the stationarity principle, or the kinetics of Briggs and Haldane, or the Bodenstein principle in chemical kinetics) and it is true that
d/dt=0
Substituting this into the differential equation, we obtain an expression for the concentration of free enzyme:
[E] \u003d (k -1 + k 2) / k 1 [S]
[E] T = [E] + = [(k -1 + k 2) / k -1 [S] + 1] =
= (k -1 + k 2 + k -1 [S]) / k 1 [S]
Steady state equation:
K 1 [S] [E] T / (k -1 + k 2 + k 1 [S])
Because v = k 2 , then we get that
v = k 1 k 2 [S] [E] T / (k -1 + k 2 + k 1 [S]) = k 2 [S] [E] T / [(k -1 + k 2) / k 1 + [S]]
In this case
V max = k 2 [E] T
and equals the maximum speed obtained from the Michaelis-Menten equation. However, the constant in the denominator of the Michaelis-Menten equation is not K S ,
those. not the dissociation constant of the enzyme-substrate complex, but the so-called Michaelis constant:
K m \u003d (k -1 + k 2) / k 1
K m is equal to K S only if .
In case, the constant in the denominator of the velocity equation is expressed by the formula
K k \u003d k 2 / k 1
and is called, according to Van Slyke, kinetic constant.
The steady state equation can also be obtained from the differential equation without the assumption that d / dt = 0. If we substitute the value [E] = [E] T - into the differential equation, after transformations we get
= (k 1 [S] [E] T - d / dt) / (k 1 [S] + k -1 + k 2)
In order to obtain the steady state equation from this equation, it does not have to be d / dt = 0. It suffices that the inequality d / dt<< k 1 [S] [E] T . Этим объясняется, почему можно достичь хорошего приближения в течение длительного времени при использовании принципа стационарности.
The differentiated steady state equation looks like this:
d / dt \u003d T / (k 1 [S] + k -1 + k 2) 2] (d [S] / dt)
This expression obviously does not equal 0.
1.2 Transformation of the Michaelis-Menten equation
The original Michaelis-Menten equation is a hyperbolic equation, where one of the constants (V max) is the asymptote to the curve. Another constant (K m), the negative value of which is determined by the second asymptote, is equal to the substrate concentration required to achieve V max / 2. This is easy to verify, since if
v=Vmax / 2, then
Vmax / 2 = Vmax [S] / (Km + [S])
V max / V max = 1 = 2 [S] / (K m + [S]) m + [S] = 2 [S], i.e. [S] = K m for v = V max /2.
The Michaelis-Menten equation can be algebraically transformed into other forms that are more convenient for graphical representation of experimental data. One of the most common transformations is simply to equate the reciprocals of the left and right sides of the equation


As a result of the transformation, we obtain the expression

which bears the name Lineweaver-Burk equations. According to this equation, a graph plotted in the coordinates 1/[S] and 1/v is a straight line, the slope of which is equal to K m /V max , and the segment cut off on the y-axis is equal to 1/V max . Such a double reciprocal graph has the advantage that it makes it possible to determine V max more precisely; on a curve plotted in [S] and v coordinates, V max is an asymptotic quantity and is determined much less accurately. The segment cut off on the x-axis on the Lineweaver-Burk plot is equal to -1/K m . Valuable information regarding enzyme inhibition can also be extracted from this graph.
Another transformation of the Michaelis-Menten equation is that both sides of the Lineweaver-Burk equation are multiplied by V max *v and after some additional transformations we get

The corresponding plot in v and v/[S] coordinates represents with e 4, fig. one]. Such a graph ( Edie-Hofsty chart) not only makes it possible to very easily determine the values of V max and K m , but also allows you to identify possible deviations from linearity that are not detected on the Lineweaver-Burk plot.
The equation can also be linearized in another form
[S] / v = K m / V max + [S] / V max
In this case, the dependence [S] / v on [S] should be built. The slope of the resulting straight line is 1 / V max ; the segments cut off on the ordinate and abscissa axes are equal to (K m / V max) and (- K m), respectively. This chart is named after the author Haynes chart.
Statistical analysis has shown that the Edie-Hofstee and Haynes methods give more accurate results than the Lineweaver-Burk method. The reason for this is that in the graphs of Edie - Hofstee and Haynes, both dependent and independent variables are included in the values plotted on both coordinate axes.
1.3 Effect of substrate concentration on reaction kinetics
In many cases, the condition of constant substrate concentration is not satisfied. On the one hand, an excess of the substrate is not used in the in vitro reaction with some enzymes due to the often occurring inhibition of the enzymatic activity of the substrate. In this case, only its optimal concentration can be used, and this does not always provide the excess of the substrate necessary to fulfill the kinetic equations of the mechanisms discussed above. Moreover, in the cell in vivo, the excess of the substrate required to fulfill this condition is usually not achieved.
In enzymatic reactions where the substrate is not in excess and, therefore, its concentration changes during the reaction, the dissociation constant of the enzyme-substrate complex is
K S = ([S] 0 - - [P]) [E] T - )/
([S] 0 - substrate concentration at t = 0). In this case, the initial reaction rate (in the steady state) is given by
v= V max / (K m + )
where is the concentration of the substrate at a point in time.
However, it is possible to write an approximate solution for two cases where [S] o = :
) if this inequality is satisfied due to large values of t, i.e. when more than 5% of the initial concentration of the substrate was consumed during the reaction;
) if the concentration of the enzyme cannot be neglected compared to the concentration of the substrate and thus the concentration of the enzyme-substrate complex must be taken into account.
If t is large and the concentration is negligible compared to [S] 0 , then the equation for the dissociation constant of the enzyme-substrate complex becomes the following:
K S = ([S] 0 - [P]) ([E] T - ) /
For the value of concentration , which changes during the reaction, the value ([S] 0 + )/2 serves as a satisfactory approximation. Since = [S] 0 - [P], the average speed; can be expressed as
![]()
Substituting this expression and the approximate value into
v= V max / (K m + ),
we get:
When comparing the values calculated on the basis of this approximation with the values obtained from the exact, integrated Michaelis-Menten equation, it turns out that the error in determining K m is 1 and 4% when spending 30 and 50% of the substrate, respectively. Therefore, the error in this approximation is negligible compared to the measurement error.
When the consumption of the substrate does not exceed 5% of the initial concentration, but the concentration of the enzyme is so high that, compared with [S] 0, it cannot be ignored, the dissociation constant of the enzyme-substrate complex is equal to:
K s = ([S] 0 - ) ([E] T - ) /
His solution regarding gives
Of the two possible solutions, only the negative one can be chosen, since only it satisfies the initial conditions: = 0 at [S] 0 = 0 or [E] T = 0. By analogy with the equation for the ratio v/V max, we have obtained the initial velocity equation. The quadratic equation obtained from the equation of the dissociation constant of the enzyme-substrate complex, found just above, using the formulas v \u003d k 2 and V max \u003d k 2 [E] T, can be reduced to the following form:
[S] 0 V max / v = K s V max / (V max - v) + [E] T
Two limiting cases should be taken into account. In the first case [S]<
v = (Vmax / Km) [S] = k[S]
Thus, we have obtained the apparent first order reaction and k=V max /K m - the apparent first order kinetic constant. Its actual dimension is time -1 , but it is a combination of the first and second order rate constants of several elementary stages, i.e. k 1 k 2 [E] T /(k -1 + k 2) . Under conditions of apparent first order k is a measure of the progress of the reaction.
Another limiting case: [S] >>
K m .
Here the constant K m
is negligible compared to [S], and thus we get v = V max .
1.4 Formation of a kinetically stable enzyme-product complex
If a kinetically stable enzyme-product complex is formed during the reaction, the reaction mechanism is as follows:
Applying the steady state assumption, we can write the differential equations:
d / dt = k 1 [E] [S] + k -2 - (k -1 + k 2) = 0 / dt = k 2 - (k -2 + k 3) = 0
From these equations it follows that
= [(k -2 + k 3) / k 2]
[E] = [(k -1 k -2 + k -1 k -3 + k 2 k 3) / k 1 k 2 [S]]
Since v = k 3
and [E] T = [E] + + =
= [(k -1 k -2 + k -1 k -3 + k 2 k 3) / k 1 k 2 [S] + (k -2 + k 3) / k 2 + 1] =
= ( (k -2 + k 3) + k 1 k 2 [S]] / k 1 k 2 [S])
we get
K 1 k 2 [S] [E] T / (k -2 + k 3 + k 2)]= k 1 k 2 k 3 [S] [E] T / (k -2 + k 3 + k 2) ]=
= [E] T [S] / [(k -1 k -2 + k -1 k -3 + k 2 k 3) / k 1 (k -2 + k 3 + k 2) + [S]]
That is
V max \u003d [E] Tm \u003d (k -1 k -2 + k -1 k -3 + k 2 k 3) / k 1 (k -2 + k 3 + k 2)
In this case, it is already very difficult to calculate the specific values of the individual rate constants, since only their ratio can be directly measured. The situation is even more complicated when the mechanism of the enzymatic reaction becomes more complex, when more than two complexes participate in the reaction, because the number of rate constants in the equation is naturally much larger, and their ratios are also more complicated.
However, the situation is simplified if, after the reversible reaction of the formation of the first complex, the subsequent elementary steps are irreversible. Important representatives of enzymes that obey this mechanism are proteolytic enzymes and esterases. Their reaction mechanism can be written as follows:
where ES` is an acyl-enzyme intermediate that decomposes on exposure to water. We can write
V max \u003d k 2 k 3 [E] 0 / (k 2 + k 3) \u003d k cat [E] 0m \u003d k 3 (k -1 + k 2) / (k 2 + k 3) k 1 cat / K m \u003d k 2 k 1 / (k -1 + k 2) \u003d k 2 / K m '
The Michaelis constant of the acylation step is K m "K s. The greater the ratio k cat / K m , the higher the specificity of the substrate.
The determination of the constants is greatly simplified if the experiment is carried out in the presence of a nucleophilic agent (N) capable of competing with water. Then

k 3 \u003d k 3 ’ and P i (i \u003d 1, 2, 3) are products.
vi = k cat, i [S] / (K m + [S]) cat, 1 = k 2 (k 3 + k 4 [N]) / (k 2 + k 3 + k 4 [N]) cat, 2 = k 2 k 3 / (k 2 + k 3 + k 4 [N]) cat, 3 = k 2 k 4 [N] / (k 2 + k 3 + k 4 [N]) m = K s ( k 3 + k 4 [N]) / (k 2 + k 3 + k 4 [N])
/v N = K s (k 3 + k 4 [N]) / k 2 k 3 [S] + (k 2 + k 3 + k 4 [N]) / k 2 k 3
Since it is known that K s / k 2 = K m / k cat, and if the nucleophile is absent, then
1/v = K s / k 2 [S] + (k 2 + k 3) / k 2 k 3
and to determine the constants, you can use the point of intersection of lines in the coordinates 1/v N (and 1/v) - 1/[S]. Two straight lines in double inverse coordinates intersect in the second quadrant. In the absence of a nucleophile, the point of intersection of the line with the vertical axis is defined as 1/V max and 1/k cat , and with the horizontal axis as -1/K m . The coordinates of the point of intersection of two lines: -1/K s and 1/k 3 . The distance between 1/V max and 1/k 3 is 1/k 2 .
1.5 Analysis of the complete reaction kinetic curve
The Michaelis - Menten equation in its original form refers only to irreversible reactions, i.e. to reactions where only the initial rate is considered, and the reverse reaction does not appear due to an insufficient amount of the product and does not affect the reaction rate. In the case of an irreversible reaction, the complete kinetic curve can be easily analyzed (for an arbitrary time interval t ),
integrating the original Michaelis-Menten equation. In this case, therefore, the assumption remains that only one intermediate enzyme-substrate complex is formed in the course of the reaction. Since for the time interval t
there are no restrictions, the concentration of the substrate at the time of analysis cannot be equal to the initially introduced concentration. Thus, it is also necessary to take into account the change in [S] during the course of the reaction. Let S 0 be the initial concentration of the substrate, (S 0 - y )
- concentration at time t .
Then, based on the original Michaelis-Menten equation (if y
is the amount of substrate converted), we can write
dy / dt \u003d V max (S 0 - y) / (K m + S 0 - y)
Taking the reciprocals and dividing the variables, we integrate over y
between 0 and y
(V max is indicated as V):
(2.303 / t) lg = V / K m - (1 / K m) (y / t)
Thus, having plotted the dependence of the left side of the equation on y / t (Foster-Niemann coordinates) ,
get a straight line with a slope (-1/K m) ,
cutoff segment on the y-axis (V/K m) ,
and on the abscissa axis - the segment V. The integral equation can also be linearized in a different way:
t / 2.3031 lg = y / 2.303 V lg + K m / V
or t/y = 2.3031 K m lg / V y +1/V
If we are studying a reversible reaction, it is necessary to pay attention to what time interval we are dealing with. At the moment of mixing of the enzyme with the substrate, the so-called pre-stationary phase begins, lasting several micro- or milliseconds, during which the enzyme-substrate complexes corresponding to the stationary state are formed. In the study of reversible reactions over sufficiently long time intervals, this phase does not play a significant role, since in this phase the reaction does not proceed at full speed in any of the directions.
For a reaction proceeding from left to right, the enzyme-substrate complexes involved in the reaction reach the rate-limiting concentration only at the end of the prestationary phase. Quasi-stationary state, in which the concentrations of the rate-determining enzyme-substrate complexes approach the maximum values of the concentrations in the steady state, lasts a few tenths of a second or a second. During this phase, the rate of product formation (or substrate consumption) is almost linear in time. Theoretically, the formation of the product has not yet occurred here, but in practice its concentration is so low that the rate of the reverse reaction does not affect the rate of the direct one. This linear phase is called the initial reaction rate, and so far we have only taken it into account.
The reaction from right to left in the next phase is also accelerated due to the gradual increase in product concentration. (transition state; the linearity observed so far in time disappears). This phase continues until the reaction rate from left to right becomes equal to the reaction rate from right to left. This is the state dynamic balance, because the reaction continues in both directions at the same rate.
2. Factors on which the rate of the enzymatic reaction depends
.1 Dependence of the enzymatic reaction rate on temperature
With an increase in the temperature of the medium, the rate of the enzymatic reaction increases, reaching a maximum at some optimal temperature, and then drops to zero. For chemical reactions, there is a rule that with an increase in temperature by 10 ° C, the reaction rate increases by two to three times. For enzymatic reactions, this temperature coefficient is lower: for every 10°C, the reaction rate increases by a factor of 2 or even less. The subsequent decrease in the reaction rate to zero indicates the denaturation of the enzyme block. The optimal temperature values for most enzymes are in the range of 20 - 40 0 C. The thermolability of enzymes is associated with their protein structure. Some enzymes are already denatured at a temperature of about 40 0 C, but most of them are inactivated at temperatures above 40 - 50 0 C. Some enzymes are inactivated by cold, i.e. at temperatures close to 0°C, denaturation occurs.
An increase in body temperature (fever) accelerates biochemical reactions catalyzed by enzymes. It is easy to calculate that an increase in body temperature for every degree increases the reaction rate by about 20%. At high temperatures of about 39-40°C, the wasteful use of endogenous substrates in the cells of a diseased organism is required to replenish their intake with food. In addition, at a temperature of about 40°C, some of the very thermolabile enzymes can be denatured, which disrupts the natural course of biochemical processes.
Low temperature causes a reversible inactivation of enzymes due to a slight change in its spatial structure, but sufficient to disrupt the corresponding configuration of the active center and substrate molecules.
2.2 Dependence of the reaction rate on the pH of the medium
For most enzymes, there is a certain pH value at which their activity is maximum; above and below this pH value, the activity of these enzymes decreases. However, not in all cases the curves describing the dependence of enzyme activity on pH are bell-shaped; sometimes this dependence can also be expressed directly. The dependence of the enzymatic reaction rate on pH mainly indicates the state of the functional groups of the active center of the enzyme. Changing the pH of the medium affects the ionization of acidic and basic groups of amino acid residues of the active center, which are involved either in the binding of the substrate (in the contact area) or in its transformation (in the catalytic area). Therefore, the specific effect of pH can be caused either by a change in the affinity of the substrate for the enzyme, or by a change in the catalytic activity of the enzyme, or both.
Most substrates have acidic or basic groups, so pH affects the degree of ionization of the substrate. The enzyme preferably binds to either the ionized or non-ionized form of the substrate. Obviously, at optimal pH, both functional groups of the active center are in the most reactive state, and the substrate is in a form that is preferable for binding by these groups of the enzyme.
When constructing curves describing the dependence of enzyme activity on pH, measurements at all pH values are usually carried out under conditions of saturation of the enzyme with the substrate, since the K m value for many enzymes changes with pH.
The curve characterizing the dependence of enzyme activity on pH can have a particularly simple shape in those cases where the enzyme acts on electrostatically neutral substrates or substrates in which charged groups do not play a significant role in the catalytic act. An example of such enzymes is papain, as well as invertase, which catalyzes the hydrolysis of neutral sucrose molecules and maintains a constant activity in the pH range of 3.0-7.5.
The pH value corresponding to the maximum activity of the enzyme does not necessarily coincide with the pH value characteristic of the normal intracellular environment of this enzyme; the latter can be both above and below the pH optimum. This suggests that the effect of pH on enzyme activity may be one of the factors responsible for the regulation of enzymatic activity within the cell. Since the cell contains hundreds of enzymes, and each of them reacts differently to changes in pH, the pH value inside the cell is perhaps one of the important elements in the complex system of regulation of cellular metabolism.
2.3 Determining the amount of the enzyme by its activity
) the total stoichiometry of the catalyzed reaction;
) the possible need for cofactors - in metal ions or coenzymes;
) the dependence of the enzyme activity on the concentrations of the substrate and cofactor, i.e. K m values for both substrate and cofactor;
) the pH value corresponding to the maximum activity of the enzyme;
) the temperature range at which the enzyme is stable and retains high activity.
In addition, it is necessary to have at your disposal some fairly simple analytical technique that allows you to determine the rate of disappearance of the substrate or the rate of appearance of the reaction products.
Whenever possible, enzyme analysis is carried out under standard conditions that maintain optimal pH and maintain a substrate concentration above saturation concentration; in this case, the initial rate corresponds to the zero order of the reaction with respect to the substrate and is proportional only to the concentration of the enzyme. For enzymes requiring cofactors, metal ions or coenzymes, the concentration of these cofactors must also exceed the saturation concentration so that the enzyme concentration is the rate-limiting factor. In general, the measurement of the rate of formation of the reaction product can be performed with greater accuracy than the measurement of the rate of disappearance of the substrate, since the substrate generally must be present in relatively high concentrations to maintain zero order kinetics. The rate of formation of the reaction product (or products) can be measured by chemical or spectrum-photometric methods. The second method is more convenient, since it allows you to continuously record the course of the reaction on the recorder's mite.
By international agreement, a unit of enzymatic activity is the amount of enzyme capable of causing the conversion of one micromole of substrate per minute at 25°C under optimal conditions. Specific activity enzyme is the number of units of enzymatic activity per 1 mg of protein. This value is used as a criterion for the purity of the enzyme preparation; it increases as the enzyme is purified and reaches its maximum value for an ideally pure preparation. Under number of revolutions understand the number of substrate molecules undergoing transformation per unit time per one molecule of the enzyme (or per one active center) under conditions where the reaction rate is limited by the concentration of the enzyme.
2.4 Enzyme activation
The regulation of enzymes can be carried out by interaction with them of various biological components or foreign compounds (for example, drugs and poisons), which are commonly called modifiers or regulators enzymes. Under the influence of modifiers on the enzyme, the reaction can be accelerated (activators) or slowed down ( inhibitors).
The activation of enzymes is determined by the acceleration of biochemical reactions that occurs after the action of the modifier. One group of activators consists of substances that affect the region of the active site of the enzyme. These include enzyme cofactors and substrates. Cofactors (metal ions and coenzymes) are not only obligatory structural elements of complex enzymes, but also essentially their activators.
Metal ions are quite specific activators. Often, some enzymes require ions of not one, but several metals. For example, for Na + , K + -ATPase, which transports monovalent cations through the cell membrane, magnesium, sodium and potassium ions are necessary as activators.
Activation with the help of metal ions is carried out by different mechanisms. In some enzymes, they are part of the catalytic site. In some cases, metal ions facilitate the binding of the substrate to the active center of the enzyme, forming a kind of bridge. Often, the metal combines not with the enzyme, but with the substrate, forming a metal-substrate complex, which is preferable for the action of the enzyme.
The specificity of the participation of coenzymes in the binding and catalysis of the substrate explains the activation of enzymatic reactions by them. The activating effect of cofactors is especially noticeable when acting on an enzyme that is not saturated with cofactors.
The substrate is also an activator within known concentration limits. After reaching saturating concentrations of the substrate, the activity of the enzyme does not increase. The substrate increases the stability of the enzyme and facilitates the formation of the desired conformation of the active site of the enzyme.
Metal ions, coenzymes and their precursors and active analogues,
substrates can be used in practice as preparations that activate enzymes.
Activation of some enzymes can be carried out by modification that does not affect the active center of their molecules. Several modifications are possible:
1) activation of an inactive predecessor - proenzyme, or zymogen. For example, the conversion of pepsinogen to pepsin ;
2) activation by attaching any specific modifying group to the enzyme molecule;
3) activation by dissociation of an inactive complex protein - active enzyme.
2.5 Enzyme inhibition
There are reagents that can interact more or less specifically with one or another side chain of proteins, which leads to inhibition of enzyme activity. This phenomenon makes it possible to study the nature of the amino acid side residues involved in this enzymatic reaction. However, in practice, one should take into account numerous subtleties that make an unambiguous interpretation of the results obtained with specific inhibitors rather difficult and often doubtful. First of all, for the reaction with an inhibitor to be suitable for studying the nature of the side chains involved in the reaction, it must satisfy the following criteria:
) be specific, i.e. the inhibitor should block only the desired groups;
) inhibit the activity of the enzyme, and this inhibition should become complete with an increase in the number of modified groups;
) the reagent should not cause non-specific denaturation of the protein.
There are 2 groups of inhibitors: reversible and irreversible action. The division is based on the criterion for the restoration of enzyme activity after dialysis or a strong dilution of an enzyme solution with an inhibitor.
According to the mechanism of action, competitive, non-competitive, non-competitive, substrate and allosteric inhibition are distinguished.
Competitive inhibition
Competitive inhibition was discovered in the study of inhibition caused by analogs of the substrate. This is the inhibition of the enzymatic reaction caused by the binding to the active center of the enzyme of an inhibitor similar in structure to the substrate and preventing the formation of an enzyme-substrate complex. In competitive inhibition, the inhibitor and substrate, being similar in structure, compete for the active site of the enzyme. The compound of molecules, which is larger, binds to the active center.
Such ideas about the mechanism of inhibition were confirmed by experiments on the kinetics of competitive inhibition reactions. Thus, it was shown that, in the case of competitive inhibition, the substrate analog does not affect the rate of decomposition of the already formed enzyme–substrate complex; when using an “infinitely large” excess of the substrate, the same maximum rate is obtained both in the presence and in the absence of an inhibitor. On the contrary, the inhibitor affects the value of the dissociation constant and the Michaelis constant. From this we can conclude that the inhibitor reacts with protein groups involved in one way or another in binding the substrate, therefore, due to its interaction with these groups, the binding strength of the substrate decreases (i.e., the number of enzyme molecules capable of binding the substrate decreases) .
Later, it was shown that kinetically competitive inhibition can be caused not only by substrate analogues, but also by other reagents, the chemical structure of which is completely different from that of the substrate. In these cases, it was also assumed that this reagent interacts with the group responsible for binding the substrate.
There are theoretically two possibilities for competitive inhibition:
1) binding and catalytic sites of the enzyme overlap; the inhibitor binds to them, but affects only the binding center groups;
2) the binding center and the catalytic center in the enzyme molecule are spatially isolated; the inhibitor interacts with the binding site.

where I is an inhibitor, and K I is the dissociation constant of the enzyme-inhibitor complex.
Relative rate (ratio of enzymatic reaction rate measured in the presence of an inhibitor (v i) ,
to the maximum speed) is equal to
v i / V = / [E] T
since for the total concentration of the enzyme it is true
[E]T = [E] + +
then 1 / v i = (K s / V[S]) (1 + [I] / K I) + 1 / V
Obviously, if [I] = K I , then the slope of the straight line becomes twice as large as for the dependence of 1/v 0 on [S] (v 0 is the rate of the enzymatic reaction in the absence of an inhibitor).
The type of inhibition is usually determined graphically. Competitive inhibition is most easily recognized by plotting Lineweaver-Burk plots (i.e. plots in 1/v i and 1/[S]) at different inhibitor concentrations. With true competitive inhibition, a set of straight lines is obtained that differ in the tangent of the slope angle and intersect the y-axis (axis 1/v i) at one point. At any concentration of the inhibitor, it is possible to use such a high concentration of the substrate that the activity of the enzyme will be maximum.
An example of competitive inhibition is the effect of various substances on the activity of succinate dehydrogenase. This enzyme is part of the enzymatic cyclic system - the Krebs cycle. Its natural substrate is succinate, and its competitive inhibitor is oxaloacetate, an intermediate product of the same Krebs cycle:
A similar competitive inhibitor of succinate dehydrogenase is malonic acid, which is often used in biochemical research.
The action of many pharmacological preparations, pesticides used to destroy agricultural pests, and chemical warfare agents is based on the principle of competitive inhibition.
For example, a group of anticholinesterase drugs, which include derivatives of quaternary ammonium bases and organophosphorus compounds, are competitive inhibitors of the cholinesterase enzyme with respect to its substrate acetylcholine. Cholinesterase catalyzes the hydrolysis of acetylcholine, a mediator of cholinergic systems (neuromuscular synapses, parasympathetic system, etc.). Anticholinesterase substances compete with acetylcholine for the active site of the enzyme, bind to it, and turn off the catalytic activity of the enzyme. Drugs such as prozerin, physostigmine, sevin inhibit the enzyme reversibly, while organophosphorus drugs such as armin, nibufin, chlorophos, soman act irreversibly, phosphorylating the catalytic group of the enzyme. As a result of their action, acetylcholine accumulates in those synapses where it is a mediator of nervous excitation, i.e. the organism is poisoned by the accumulated acetylcholine. The action of reversible inhibitors gradually disappears, since the more acetylcholine accumulates, the faster it displaces the inhibitor from the active center of cholinesterase. The toxicity of irreversible inhibitors is incomparably higher; therefore, they are used to combat agricultural pests, household insects and rodents (for example, chlorophos) and as chemical warfare agents (for example, sarin, soman, etc.).
Noncompetitive inhibition
In non-competitive inhibition, a specific inhibitor does not affect the dissociation constant of the enzyme-substrate complex. On the other hand, the maximum achievable reaction rate is lower in the presence of an inhibitor than in its absence, even at an infinitely large excess of the substrate. The presence of inhibition proves that the inhibitor binds to the protein. The invariance of the dissociation constant both in the presence and absence of the inhibitor, in turn, indicates that, unlike the substrate, the inhibitor binds to another group. From a theoretical point of view, the mechanism of such inhibition can be interpreted in various ways.
a) The binding site and the catalytic site of the enzyme are different. In this case, the inhibitor associated with the catalytic center reduces the activity of the enzyme and the maximum achievable
speed without affecting the formation of the enzyme-substrate complex.
b) The binding site and the catalytic site overlap on
surface of the enzyme, and the inhibitor binds to other groups of the protein. Due to the binding of the inhibitor to the surface of the enzyme, the information of the protein changes and becomes unfavorable for the implementation of catalysis.
c) The inhibitor does not bind to either the catalytic site or the binding site, and thus does not affect the conformation of the protein. However, it can locally change the charge distribution on a region of the protein surface. Inhibition of activity can also occur in this case if, for example, ionization of groups essential for the manifestation of activity is made impossible, or if, on the contrary, ionization of groups active only in non-ionized form occurs. This phenomenon is observed mainly when using strongly acidic or strongly alkaline reagents.
The inhibitor and the substrate do not affect each other's binding to the enzyme, but the enzyme complexes containing the inhibitor are completely inactive. In this case, the following elementary stages can be assumed:

v i / V = / [E] T
[E] T = [E] + + +
/ v i = (K s / V [S]) (1 + [I] / K I) + (1 / V) (1 + [I] / K I)
If [I] = K I, the slopes of the straight lines and the ordinates of the point of intersection with the vertical axis are doubled compared to 1/v 0 .
Non-competitive inhibitors are, for example, cyanides, which are strongly associated with ferric iron, which is part of the catalytic site of the hemin enzyme - cytochrome oxidase. Blockade of this enzyme turns off the respiratory chain, and the cell dies. Non-competitive enzyme inhibitors include heavy metal ions and their organic compounds. Therefore, heavy metal ions of mercury, lead, cadmium, arsenic and others are very toxic. They block, for example, SH-groups included in the catalytic site of the enzyme.
Non-competitive inhibitors are cyanides, which are strongly associated with ferric iron, which is part of the catalytic site of the hemic enzyme - cytochrome oxidase. Blockade of this enzyme turns off the respiratory chain, and the cell dies. It is impossible to remove the action of a non-competitive inhibitor with an excess of the substrate (as the action of a competitive one), but only with substances that bind the inhibitor - reactivators.
Non-competitive inhibitors are used as pharmacological agents, toxic substances for pest control in agriculture and for military purposes. In medicine, preparations containing mercury, arsenic, bismuth are used, which non-competitively inhibit enzymes in the cells of the body or pathogenic bacteria, which determines one or another of their effects. In case of intoxication, the binding of poison or its displacement from the enzyme-inhibitor complex is possible with the help of reactivators. These include all SH-containing complexones (cysteine, dimercaptopropanol), citric acid, ethylenediaminetetraacetic acid, etc.
Uncompetitive inhibition
This type of inhibition is also called anticompetitive inhibition in the literature. or associated inhibition , however, the term "uncompetitive inhibition" is the most widely used. The characteristic of this type of inhibition is that the inhibitor is not able to attach to the enzyme, but it does attach to the enzyme-substrate complex.
In the case of uncompetitive inhibition, the complex containing the inhibitor is inactive:

v i / V = / [E]
[E]T = [E] + +
/ v i = Ks / V[S] + (1 / V) (1 + [I] / K I)
substrate inhibition
Substrate inhibition is the inhibition of an enzymatic reaction caused by an excess of substrate. Such inhibition occurs due to the formation of an enzyme-substrate complex that is not capable of undergoing catalytic transformations. The ES 2 complex is unproductive and makes the enzyme molecule inactive. Substrate inhibition is caused by an excess of the substrate, therefore, it is removed when its concentration decreases.
Allosteric inhibition
Allosteric regulation is characteristic only for a special group of enzymes with a quaternary structure, which have regulatory centers for binding allosteric effectors. Negative effectors that inhibit the conversion of the substrate in the active site of the enzyme act as allosteric inhibitors. Positive allosteric effectors, on the contrary, accelerate the enzymatic reaction, and therefore they are referred to as allosteric activators. Allosteric effectors of enzymes are most often various metabolites, as well as hormones, metal ions, and coenzymes. In rare cases, substrate molecules play the role of an allosteric effector of enzymes.
The mechanism of action of allosteric inhibitors on the enzyme is to change the conformation of the active site. The decrease in the rate of the enzymatic reaction is either a consequence of an increase in K m or a decrease in the maximum rate V max at the same saturating substrate concentrations, i.e. the enzyme is partially idle.
Allosteric enzymes differ from other enzymes in their specific S-shaped curve of reaction rate versus substrate concentration. This curve is similar to the oxygen saturation curve of hemoglobin; it indicates that the active centers of the subunits do not function autonomously, but cooperatively, i.e. the affinity of each next active center for the substrate is determined by the degree of saturation of the previous centers. The coordinated work of the centers is determined by allosteric effectors.
Allosteric regulation manifests itself in the form of inhibition by the end product of the first enzyme in the chain. The structure of the final product after a series of transformations of the starting substance (substrate) is not similar to the substrate, so the final product can act on the initial enzyme of the chain only as an allosteric inhibitor (effector). Outwardly, such regulation is similar to the regulation by the feedback mechanism and allows you to control the output of the final product, in the event of accumulation of which the work of the first enzyme in the chain stops. For example, aspartate carbamoyltransferase (ACTase) catalyzes the first of six reactions in the synthesis of cytidine triphosphate (CTP). CTP is an allosteric AKTase inhibitor. Therefore, when CTP accumulates, AKTase inhibition occurs and further CTP synthesis stops. Allosteric regulation of enzymes with the help of hormones has been discovered. For example, estrogens are an allosteric inhibitor of the enzyme glutamate dehydrogenase, which catalyzes the deamination of glutamic acid.
Thus, even the simplest kinetic equation for an enzymatic reaction contains several kinetic parameters, each of which depends on the temperature and environment in which the reaction takes place.
Inhibitors make it possible not only to understand the essence of enzymatic catalysis, but also are a kind of tool for studying the role of individual chemical reactions, which can be specifically switched off with the help of an inhibitor of a given enzyme.
3. Some devices useful for determining initial reaction rates
Many problems of enzymatic kinetics lead to the determination of the initial reaction rates (v 0). The main advantage of this method is that the values of v0 determined at the initial moment of time will give the most accurate representation of the activity of the enzymes under study, since the accumulating reaction products do not yet have time to exert an inhibitory effect on the enzyme and, in addition, the reacting system is in a state of stationary equilibrium. .
In laboratory practice, however, when using conventional spectrophotometric, titrimetric, or other techniques for recording the progress of such reactions, at best, up to 15–20 seconds from the initial time for introducing the enzyme to the substrate, mixing the reacting system, setting up the cell, etc. is lost. And this is unacceptable, since in this case the tangent is brought to the point where tg ά 2< tg ά 1 . Не компенсируется потеря начального времени и при математической обработке таких кривых при записи выхода v 0 на максимальный уровень (V). Кроме того, протекание реакций без constant mixing is further complicated by fluctuations in the concentrations of reagents by volume.
The simple devices proposed below for a spectrophotometer, a pH meter, and the like make it possible to significantly reduce the sources of the indicated errors in determining v 0 .
3.1 Device to the spectrophotometer
The device for the spectrophotometer consists of a dispenser 1, a rotating Teflon thread 2 (a stirrer) and a fixing cap 3.
The dispenser is a micropipette, one end of which is formed with a needle 4, the other - with a widening 5 (to prevent the enzyme from entering the rubber tip 6).
The Teflon cover 3 covering the spectral cuvette 7 has two holes: one (8) in the center of the cover, the second (9) above the middle of the gap between the opaque wall of the cuvette 7 and the light beam 10. Teflon tube 11 (inner diameter 1 -1.5 mm) is fixed at one end in hole 9, the other - on a fixed ledge 12 in front of the motor rotor 13. Teflon thread 2 is inserted inside the tube (thread thickness 0.5-0.6 mm). One end of the thread is fixed on the rotating rotor of the motor 13, the second - passed into the cuvette 7 - is shaped in the form of a spiral (to enhance mixing). The position of the thread is determined by the fixing cap 3, regardless of the distance of the motor, which is convenient when working requiring frequent changes of cuvettes.
Principle of operation. The quartz cuvette of the spectrophotometer 7 is filled with substrate 14 (about 1.5-2.0 ml), inserted into the thermostatic cuvette holder of the spectrophotometer, closed with a lid 3 with a rotating Teflon thread 2, which is immersed in the substrate 14, and all further operations are performed already in the beam of light of the spectrophotometer and recorded on the recorder.
At the beginning of work, the substrate is mixed, and the pen of the recorder writes a flat horizontal (or “zero”) line. The dispenser (with the enzyme) is inserted into hole 8 (the needle is immersed in the substrate solution 14), by quickly squeezing the tip 6, the enzyme (usually about 0.03-0.05 ml) is introduced into the substrate, and the dispenser is removed. The mixing of the components ends in 2.5-3 s, and the pen of the recorder fixes the beginning of the reaction by deviating the curve of optical density (ΔA) versus time.
Such a device also makes it possible to take samples from the reacting system for analysis; add inhibitors and activators to the system; change the reaction conditions (change the pH, ionic strength, etc.) without disturbing the registration of the reaction course, which is very convenient, for example, when studying the splitting n-NFF "acidic" phosphatases, where cleavage n-NFF is carried out at pH 5.0 (or pH 6-7), and the activity of enzymes is determined by the accumulation n-nitrophenolate ions at pH 9.5-10.0.
Such a device is also convenient for carrying out spectrophotometric titration of enzymes, etc.
3.2 Device for pH meter
The device for the pH meter consists of a modified tip of the flow electrode 1, a semi-microcell 2, a dispenser 3, and an electronic circuit for connecting the pH meter to the recorder. In addition, the device includes a standard pH meter electrode (4), a cell holder cover (5), a thermostatic flow chamber (6), a substrate solution (7), a passive magnet (8), and an active magnet (9).
The standard tip of the flow electrode of the pH meter (LPU-01) is replaced by a Teflon tube 1 (inner diameter 1.3-1.5 mm), filled with asbestos thread, pre-treated with a saturated KCl solution. The filling density of the thread is controlled so that the flow rate of the KCl solution through the tube is close to the flow rate of the original unmodified electrode. This replacement of the tip makes it possible to reduce the size of the original working cell from 20–25 to 2 ml, which makes it possible to manage with minimal volumes (1.5 ml) of solutions of expensive biochemical preparations.
The electronic circuit for connecting the pH meter (LPU-01) to the recorder consists of a power source (DC battery 12 V), a variable wire resistance R 1 (10 - 100 Ohm), which sets a voltage of 9 V at the D809 zener diode according to the voltmeter reading, a variable wire resistance R 2 (15-150 Ohm), which regulates the setting of the "zero" (reference point) of the pH meter readings on the scale of the recorder, and variable wire resistance R 3 (35-500 Ohm), which regulates the scale of expansion (amplification) of the readings of the pH scale - meters on the recorder. The circuit operates reliably until the source voltage drops below 9 V.
Principle of operation. 1.5 ml of the substrate is introduced into the cell (a glass cylinder 1.7x2.4 cm), and the cell is fixed on the fixing cap 5. Stirring 9 is turned on, and the pen of the recorder writes an even (basic) reference line. With the help of a dispenser, 0.03 ml of the enzyme solution is introduced into the substrate, and the pen of the recorder fixes the beginning of the reaction by deviating the curve of pH versus time (t).
Such a device does not replace a pH stat, but taking into account the possibility of expanding the pH meter scale, it allows you to reliably record minor changes in pH 0.004-0.005.
3.3 Nomogram rulers, convenient for determining the initial speed
Considerable complexity of determining the initial velocity in the method of tangents is the calculation of the ratios of changes in the concentrations of reagents (Δ[S]) per unit time (Δt), i.e. expression v 0 in M/min from the conditions that
v 0 = lim Δ[S] / Δt, at, t 0.
In practice, such a procedure usually consists of three or four separate operations: a tangent is drawn to the initial section of the reaction curve, then the number of units of the registered value (optical density, rotation angle, etc.) per a certain time interval is counted, and this leads to unit of time and, finally, recalculate the readings of the recorder for the change in the concentration of the reagent for 1 min (M/min). The proposed two types of nomogram ruler make it possible to simplify this procedure.
Rectangular ruler. v 0 is the ratio Δ[S]/Δt, i.e. tg ά, where ά is the angle of inclination of the tangent to the time axis t. The same tangent is also the hypotenuse of the corresponding right triangle with legs [S] and t. The larger v 0 , the steeper the slope of the tangent. Therefore, if we limit ourselves to a certain time interval, for example 1 min, then we will get a series of right-angled triangles with different values of the leg [S] (actually, different values of v 0). If, however, both legs are graduated: horizontal - in units of time reference (1 min), and vertical - in units of change in reagent concentrations, for example, in millimoles (mM), and apply the resulting segments to a suitable format from a transparent material (plexiglass about 2 mm thick) , then you can get a convenient ruler for determining the initial reaction rates. All numbers and lines are printed on the reverse side of the ruler to eliminate parallax errors when determining v 0 .
The procedure for determining v 0 is reduced in this case to two simple operations: a tangent is drawn to the initial section of the kinetic curve t 2 and combine the zero point of the horizontal leg t of the ruler with the beginning of the tangent, the continuation of the tangent will now cross the concentration scale [S] at the point that determines the value of v 0 in M/min (when the leg t is horizontal, no additional operations are required.
Arc line. The procedure for determining v 0 can be simplified to one operation if the concentration scale is plotted along an arc of a certain radius.
A straight ("basic") line 2 is applied to a plate of transparent material (all numbers and lines are also applied on the reverse side of the ruler) and from the zero point (t=0, min) of this line with a radius equal to the leg length t=1 min [ , draw an arc [S], from top to bottom along which the scale of changes in the concentrations of the reagent (for example, substrate in mM) is laid.
The described types of rulers, a device for a spectrophotometer and a pH meter have been used for a number of years to determine the initial reaction rates (v 0), in the study of the substrate specificity of enzymes, for spectrophotometric titration, etc.
Conclusion
In this paper, a section of enzymology was considered that studies the dependence of the rate of chemical reactions catalyzed by enzymes on a number of environmental factors. The founders of this science are considered to be Michaelis and Menten, who published their theory of the general mechanism enzymatic reactions, derived an equation that has become the fundamental principle of all kinetic studies of enzymes, it serves as a starting point for any quantitative description of the action of enzymes. The original Michaelis-Menten equation is a hyperbolic equation; Lineweaver and Burke contributed to the kinetics by transforming the Michaelis-Menten equation and obtaining a graph of a straight line from which the value of V max can be most accurately determined.
Over time, the change in the rate of the enzymatic reaction in the enzymatic reaction under experimental conditions decreases. A decrease in the rate can occur due to a number of factors: a decrease in the concentration of the substrate, an increase in the concentration of a product that can have an inhibitory effect, changes in the pH of the solution, and changes in the temperature of the medium can occur. Thus, for every 10°C rise in temperature, the reaction rate doubles or even less. Low temperature reversibly inactivates enzymes. The dependence of the rate of the enzymatic reaction on pH indicates the state of the functional groups of the active center of the enzyme. Each enzyme reacts differently to changes in pH. Chemical reactions can be stopped by acting on them with various types of inhibition. The initial reaction rate can be quickly and accurately determined with the help of such devices as nomogram rulers, a spectrophotometer device and a pH meter. This allows the most accurate representation of the activity of the studied enzymes.
All this is actively used today in medical practice.
List of sources used
1. Belyasova N.A. Biochemistry and molecular biology. - Minsk: book house, 2004. - 416 p., ill.
Keleti T. Fundamentals of enzymatic kinetics: Per. from English. - M.: Mir, 1990. -350 p., ill.
3. Knorre D.G. Biological chemistry: Proc. for chemical, biol. and honey. specialist. universities. - 3rd ed., Rev. - M.: Higher. school 2002. - 479 p.: ill.
4. Krupyanenko V.I. Vector method for representing enzymatic reactions. - M.: Nauka, 1990. - 144 p.
5. Lehninger A. Biochemistry. Molecular bases of the structure and function of the cell: Per. from English. - M.: Mir, 1974.
6. Stroev E.A. Biological Chemistry: A Textbook for Pharmacy. in-tov and pharmac. fak. honey. in-comrade. - M.: Higher school, 1986. - 479 p., ill.
Severin E.S. Biochemistry. a. - 5th ed. - M.: GEOTAR - Media, 2009. - 786 p., ill.
This section of enzymology studies the influence of various factors on the rate of an enzymatic reaction. Taking into account the general equation of enzymatic catalysis of the reversible reaction of the transformation of one substrate into one product (1),
the main factors influencing the rate of an enzymatic reaction should be named: substrate concentration [S], enzyme concentration [E], and reaction product concentration [P].
The interaction of some enzymes with their substrate can be described by a hyperbolic curve of the dependence of the enzymatic reaction rate V on the concentration of the substrate [S] (Fig. 19):

Fig. 19. Dependence of the rate of the enzymatic reaction on the concentration of the substrate.
Three segments can be distinguished on this curve, which can be explained by the positions of the mechanism of interaction of the enzyme with the substrate: OA is the segment of the directly proportional dependence of V on [S], the active sites of the enzyme are gradually filled with substrate molecules with the formation of an unstable complex ES; section AB - curvilinear dependence of V on [S], complete saturation of the active centers of the enzyme with substrate molecules has not yet been achieved. The ES complex before reaching the transition state is unstable, the probability of back dissociation to E and S is still high; section BC - dependence is described by a zero-order equation, the section is parallel to the [S] axis, complete saturation of active enzymes with substrate molecules is achieved, V=V max .
The characteristic shape of the curve is described mathematically by the Briggs-Haldane equation:
V=V max ● [S]/ Km + [S] (2),
where Km is the Michaelis-Menten constant, numerically equal to the substrate concentration at which the rate of the enzymatic reaction is equal to half V max .
The lower the K m of the enzyme, the higher the affinity of the enzyme for the substrate, the faster the transition state for the substrate is reached, and it turns into a reaction product. The search for Km values for each of the enzyme's substrates with group specificity is important in determining the biological role of this enzyme in the cell.
For most enzymes, it is impossible to construct a hyperbolic curve (Fig. 19). In this case, the double reciprocal method (Lineweaver-Burk) is used, i.e. a graphical dependence of 1/[V] on 1/[S] is plotted (Fig. 20). The method of constructing such curves in an experiment is very convenient when studying the effect of various types of inhibitors on the activity of enzymes (see the text below).

Fig.20. Plot of 1/[V] versus 1/[S] (Lineweaver-Burk method),
where y-cut-off area - , and x – cut-off area -  , the tangent of the angle α - .
, the tangent of the angle α - .
Dependence of the enzymatic reaction rate V on the enzyme concentration [E].
This graphical dependence (Fig. 21) is considered at the optimum temperature and pH of the environment, at substrate concentrations that are significantly higher than the saturation concentration of the active sites of the enzyme.

Rice. 21. Influence of enzyme concentration on the rate of enzymatic reaction.
Dependence of the enzymatic reaction rate on the concentration of the cofactor or coenzyme. For complex enzymes, it should be borne in mind that the deficiency of coenzyme forms of vitamins in hypovitaminosis, a violation of the intake of metal ions into the body necessarily lead to a decrease in the concentration of the corresponding enzymes necessary for the course of metabolic processes. Therefore, it should be concluded that the activity of the enzyme is directly dependent on the concentration of the cofactor or coenzyme.
Influence of the concentration of products on the rate of the enzymatic reaction. For reversible reactions occurring in the human body, it must be taken into account that the products of the direct reaction can be used by the enzyme as substrates for the reverse reaction. Therefore, the direction of the flow and the moment of reaching V max are dependent on the ratio of the concentrations of the initial substrates and reaction products. For example, the activity of alanine aminotransferase, which catalyzes the transformation:
Alanine + Alpha-ketoglutarate ↔ Pyruvate + Glutamate
depends in the cell on the ratio of concentrations:
[alanine + alpha-ketoglutarate] / [pyruvate + glutamate].
MECHANISM OF ENZYME ACTION. THEORIES OF ENZYMATIVE CATALYSIS
Enzymes, like non-protein catalysts, increase the rate of a chemical reaction due to their ability to lower the activation energy of that reaction. The activation energy of an enzymatic reaction is calculated as the difference between the energy value in the system of the ongoing reaction that has reached the transition state and the energy determined at the beginning of the reaction (see the graphic dependence in Fig. 22).

Rice. 22. Graphical dependence of the energy state of a chemical reaction without an enzyme (1) and in the presence of an enzyme (2) on the time of the reaction.
The works of V. Henry and, in particular, L. Michaelis, M. Menten on the study of the mechanism of monosubstrate reversible enzymatic reactions made it possible to postulate that the enzyme E first reversibly and relatively quickly combines with its substrate S to form an enzyme-substrate complex (ES):
E+S<=>ES (1)
The formation of ES occurs due to hydrogen bonds, electrostatic, hydrophobic interactions, in some cases covalent, coordination bonds between the side radicals of the amino acid residues of the active center and the functional groups of the substrate. In complex enzymes, the non-protein part of the structure can also perform the function of contact with the substrate.
The enzyme-substrate complex then breaks down in a second slower reversible reaction to form the reaction product P and the free enzyme E:
ES<=>EP<=>E + P (2)
At present, thanks to the work of the above-mentioned scientists, as well as Kaylin D., Chance B., Koshland D. (the theory of "induced conformity"), there are theoretical provisions on four main points in the mechanism of enzyme action on the substrate, which determine the ability of enzymes to accelerate chemical reactions. reactions:
1. Orientation and proximity . The enzyme is able to bind the substrate molecule in such a way that the bond attacked by the enzyme is not only located in the immediate vicinity of the catalytic group, but also correctly oriented with respect to it. The probability that the ES complex will reach the transition state due to orientation and approach is greatly increased.
2. Stress and strain : induced fit. Attachment of a substrate can cause conformational changes in the enzyme molecule, which lead to tension in the structure of the active site, and also somewhat deform the bound substrate, thereby facilitating the achievement of the transition state by the ES complex. There is a so-called induced correspondence between E and S molecules.
Enzymatic kinetics studies the rate of reactions catalyzed by enzymes depending on various conditions (concentration, temperature, pH, etc.) of their interaction with the substrate.
However, enzymes are proteins that are sensitive to the influence of various external influences. Therefore, when studying the rate of enzymatic reactions, mainly the concentrations of reactants are taken into account, and the influence of temperature, pH of the medium, activators, inhibitors, and other factors is tried to be minimized and standard conditions are created. First, this is the optimal pH value of the medium for this enzyme. Secondly, it is recommended to keep the temperature at 25°C, where possible. Thirdly, complete saturation of the enzyme with the substrate is achieved. This point is especially important, since at a low substrate concentration, not all enzyme molecules participate in the reaction (Fig. 6.5, a), which means that the result will be far from the maximum possible. The highest power of the catalyzed reaction, other things being equal, is achieved if each enzyme molecule is involved in the transformation, i.e. at a high concentration of the enzyme-substrate complex (Fig. 6.5, v). If the concentration of the substrate does not ensure complete saturation of the enzyme (Fig. 6.5, b), then the rate of the proceeding reaction does not reach the maximum value.
Rice. 65.
a - at low substrate concentration; 6 - with insufficient concentration of the substrate; v - when the enzyme is completely saturated with the substrate
The rate of an enzymatic reaction, measured under the above conditions, and the complete saturation of the enzyme with the substrate is called maximum rate of enzymatic reaction (V).
The rate of the enzymatic reaction, determined when the enzyme is not completely saturated with the substrate, is denoted v.
Enzymatic catalysis can be simplistically described by the scheme

where F is an enzyme; S - substrate; FS - enzyme-substrate complex.
Each stage of this process is characterized by a certain speed. The unit for measuring the rate of an enzymatic reaction is the number of moles of the substrate converted into a unit of time(like the rate of a normal reaction).
The interaction of the enzyme with the substrate leads to the formation of an enzyme-substrate complex, but this process is reversible. The rates of the forward and reverse reactions depend on the concentrations of the reactants and are described by the corresponding equations:
Equation (6.3) is valid in equilibrium, since the rates of the forward and reverse reactions are equal.
Substituting the rates of direct (6.1) and reverse (6.2) reactions into equation (6.3), we obtain the equality:
The state of equilibrium is characterized by the corresponding equilibrium constant K p, equal to the ratio of the constants of direct and reverse reactions (6.5). The reciprocal of the equilibrium constant is called substrate constant K s , or the dissociation constant of the enzyme-substrate complex:

From equation (6.6) it is clear that the substrate constant decreases at a high concentration of the enzyme-substrate complex, i.e. with great stability. Therefore, the substrate constant characterizes the affinity of the enzyme and the substrate and the ratio of the rate constants for the formation and dissociation of the enzyme-substrate complex.
The phenomenon of saturation of an enzyme with a substrate was studied by Leonor Michaelis and Maud Mepten. Based on the mathematical processing of the results, they derived equation (6.7), which received their names, from which it is clear that at a high concentration of the substrate and a low value of the substrate constant, the rate of the enzymatic reaction tends to the maximum. However, this equation is limited because it does not take into account all parameters:
The enzyme-substrate complex during the reaction can undergo transformations in different directions:
- dissociate into the original substances;
- be converted into a product from which the enzyme is separated unchanged.
Therefore, to describe the overall effect of the enzymatic process, the concept Michaelis constants K t, which expresses the relationship of the rate constants of all three reactions of enzymatic catalysis (6.8). If both terms are divided by the rate constant of the reaction of formation of the enzyme-substrate complex, then the expression (6.9) will be obtained:

An important corollary follows from equation (6.9): the Michaelis constant is always greater than the substrate constant by k 2 /kv
Numerically K t is equal to such a concentration of the substrate at which the reaction rate is half the maximum possible rate and corresponds to such saturation of the enzyme with the substrate, as in Fig. 6.5, b. Since in practice it is not always possible to achieve complete saturation of the enzyme with the substrate, it is K t used to compare the kinetic characteristics of enzymes.
The rate of the enzymatic reaction in the case of incomplete saturation of the enzyme with the substrate (6.10) depends on the concentration of the enzyme-substrate complex. The coefficient of proportionality is the reaction constant for the release of the enzyme and the product, since this changes the concentration of the enzyme-substrate complex:
After the transformations, taking into account the dependencies presented above, the rate of the enzymatic reaction in the case of incomplete saturation of the enzyme with the substrate is described by equation (6.11), i.e. depends on the concentrations of the enzyme, substrate and their affinity Ks:
The graphical dependence of the rate of the enzymatic reaction on the concentration of the substrate is not linear. As is obvious from Fig. 6.6, with an increase in the concentration of the substrate, an increase in the activity of the enzyme is observed. However, when the maximum saturation of the enzyme with the substrate is reached, the rate of the enzymatic reaction becomes maximum. Therefore, the factor limiting the reaction rate is the formation of an enzyme-substrate complex.
Practice has shown that the concentrations of substrates, as a rule, are expressed in values much less than unity (10 6 -10 3 mol). It is quite difficult to operate with such quantities in the calculations. Therefore, G. Lineweaver and D. Burke proposed to express the graphical dependence of the rate of the enzymatic reaction not in direct coordinates, but in inverse ones. They proceeded from the assumption that for equal values, their reciprocals are also equal:

Rice. 6.6.
After the transformation of expression (6.13), an expression is obtained, called Lineweaver-Burk equation (6.14):
The graphical dependence of the Lineweaver-Burk equation is linear (Fig. 6.7). The kinetic characteristics of the enzyme are determined as follows:
- the segment cut off on the y-axis is equal to 1/V;
- the segment cut off on the x-axis is -1 /K t.

Rice. 6.7.
It is believed that the Lineweaver - Burke method allows you to more accurately determine the maximum reaction rate than in direct coordinates. Valuable information regarding enzyme inhibition can also be extracted from this graph.
There are other ways to transform the Michaelis-Menten equation. Graphic dependencies are used in studying the influence of various external influences on the enzymatic process.