Chemistry and chemical technology
Articles Pictures Tables About site РусскийHydrogen peroxide decomposition catalysts
The decomposition of peroxide compounds occurs in the presence of certain metals (iron, copper, manganese, cobalt, chromium) and their salts, which are catalysts. Therefore, concentrated hydrogen peroxide, peracetic acid, and a number of other peroxides are capable of exploding in the absence of organic substances.Practical application as a single-component fuel has found 80-90% hydrogen peroxide. It was used in the V-2 rocket as an auxiliary fuel for the formation of a naro-gas mixture, which drives the turbines of the pumps that supply fuel to the rocket engine. The decomposition of hydrogen peroxide is carried out using solid or liquid catalysts.
Lead is one of the most active heterogeneous catalysts. Various qualitative characteristics of this catalytic process have been published, namely, divalent lead in an acidic solution has no effect on hydrogen peroxide; for its decomposition, it requires an alkaline medium in which lead dioxide is formed. As a result of studying the mechanism of this catalysis, it was concluded that it can be described as a redox cycle between divalent lead Pb(OH). and red lead PbzO. Conditions of high catalytic activity occur when both of these substances are present as solid phases in a strongly alkaline solution, higher oxides are formed. The effect of different pH ranges can be characterized as follows. Lead nitrate dissolves in hydrogen peroxide to form clear, stable solutions. When alkali is added, a whitish-yellow precipitate forms and little activity occurs. With further addition of alkali, the precipitate turns orange-red and the rapid decomposition of peroxide begins. As it turned out, the amount of alkali required to reach this point is inversely proportional to the amount of dissolved lead, superimposed on this phenomenon is not yet clearly established the influence of aging. The amount of pyrophosphate required to terminate catalysis is approximately equivalent to that required to form lead pyrophosphate PbPO. Catalytic activity peaks at approximately 0.2N. alkali concentration at a higher concentration, the solubility of lead in the form of plumbite and plumbate increases and the catalytic activity decreases. An attempt was made to prove the existence of a cyclic oxidation-reduction process using radioactive tracers, but it ended in failure due to the fact that even in the absence of hydrogen nonoxide, an exchange occurs between the divalent lead ion and lead dioxide in nitric acid (which corresponds to the literature data) and between plumbate and plumbate in the main solution (which contradicts the published data
The effect of increasing the surface area of a catalyst on its catalytic activity can be illustrated by an example. Hydrogen peroxide can decompose into water and oxygen. The catalyst for this process is platinum. On a smoothly polished platinum surface, the H2O2 decomposition reaction is almost not accelerated. On a rough surface, a weak release of oxygen is observed. Powdered platinum rather quickly decomposes hydrogen peroxide on platinum black; the process is very vigorous, and the addition of a colloidal solution of platinum leads to a violent reaction, sometimes accompanied by an explosion.
Lead compounds are active decomposition catalysts. Lead equipment can only be successfully used in conditions where sulfates are present, causing the formation of an inert lead sulfate coating. Lead is used in some cases in plants producing hydrogen peroxide by the electrolytic peroxodisulfate process and in the bleaching of raw wool in the presence of sulfates. However, the use of lead in contact with all but very dilute peroxide solutions can be hazardous and should therefore be avoided.
When storing hydrogen peroxide solutions, negative catalysts are used to prevent its decomposition. As such catalysts, called stabilizers, small amounts of phosphoric acid, salicylic, uric acids can be used (for example, 1 g of uric acid is enough for 30 liters of concentrated peroxide), which protect hydrogen peroxide from decomposition.
Solid hydrogen peroxide is unusually inert. For example, if chilled 0.5 n. permanganate solution, rust particles or other catalysts and do not allow the peroxide to thaw, decomposition is not observed at all. Decomposition begins only after melting.
The purer the hydrogen peroxide, the slower it decomposes during storage. Particularly active catalysts for the decomposition of H2O2 are compounds of certain metals (Cp, Fe, Mn, etc.), and even traces of them that cannot be directly determined analytically act noticeably. To bind these metals to hydrogen peroxide as a stabilizer, a little (on the order of 1 10 000) sodium pyrophosphate - N34P207 is often added.
The steam generator is a chamber in which the catalyst is placed. Hydrogen peroxide is fed into the chamber, where it decomposes into water vapor and oxygen, and heat is released. A liquid catalyst can also be used to decompose hydrogen peroxide. In this case, the chamber of the steam-gas generator is a chamber for mixing liquid catalyst with hydrogen peroxide and decomposition of the latter.
Very concentrated (80% and more) aqueous solutions of H2O2 are used as energy sources and independently (using catalysts for the rapid decomposition of H2O2 from one liter of liquid hydrogen peroxide, you can get about 5000 liters of a mixture of oxygen heated to 700 ° C with water vapor), and as an oxidizer for jet fuels. Hydrogen peroxide is also used as an oxidizing agent in chemical industries, as a feedstock for the production of many peroxide compounds, as an initiator of polymerization processes, and in the manufacture of some porous products. for artificial aging of wines, hair dyeing, stain removal, etc.
Hydrogen peroxide is widely used to produce steam and gas for the operation of a turbopump unit of a rocket propulsion system. On the one hand, the fuel that ensures the operation of HPP is required to have sufficiently high energy performance to ensure the operation of pumps at minimum costs, on the other hand, to have a relatively low combustion temperature. The most widespread as a single-component fuel for driving a turbopump unit is 80-85% hydrogen peroxide. When 80% hydrogen peroxide is decomposed, steam gas is obtained with a temperature of 450-500 ° C. In addition to peroxide, a catalyst is consumed when producing steam gas. For the decomposition of one kilogram of peroxide, 0.05 kg of a liquid catalyst is consumed, which is a 35% alcohol solution of KaMnO4 (sodium permanganate).
Matheson and Maas determined the heat of decomposition of 10-gram solutions of hydrogen peroxide in an adiabatic calorimeter. Manganese dioxide was used as a decomposition catalyst. According to these authors, decomposition ended abruptly, and the authors did not introduce corrections for residual hydrogen peroxide. A correction was made for water vapor and a certain part of the water equivalent of the calorimeter was calculated. By linear extrapolation of the heat of dilution, based on the average of four determinations (two determinations with 38.05% peroxide and two with 97.15% peroxide), the heat of decomposition of anhydrous hydrogen nonoxide (-23.45 kcal/mol) is calculated .
Decomposition reactions are characteristic of propellant components, which are endothermic substances. As a rule, they can be stored for a long time without decomposition at normal temperatures, but with an increase in temperature or under the influence of a catalyst, decomposition begins, self-accelerating under the influence of the heat released. Thus, hydrazine, heated to 350°C, completely decomposes into nitrogen and ammonia, and much more intensively in the presence of oxides of iron, chromium, copper, and other catalysts. Hydrogen peroxide is a characteristic substance capable of decomposing with the release of heat. In its pure form, it is quite stable and only when heated above 140 ° C does it begin to decompose into water and oxygen with the release of heat. Absolutely pure H2O2 can be heated to boiling (151.4°C) and distilled without decomposition, but even the slightest scratches on the walls of the vessel in which hydrogen peroxide is heated can cause its decomposition. The rate of peroxide decomposition depends on its concentration, pH value, temperature, nature and amount of impurities or stabilizers catalyzing the decomposition, physical and chemical nature of the surface of the vessels containing H2O2.
Elemental carbon does not enter into a stoichiometric reaction with hydrogen peroxide, although the decomposition that occurs in this case causes a certain change in the surface of the carbon. Roop and Schlee reported that hydrogen peroxide oxidizes carbonate to formic acid and formaldehyde, later
Zinc has the unusual properties of being able to function as both a catalyst and a stabilizer. As indicated on page 451, zinc in a solution of 90% hydrogen peroxide has a stabilizing effect. It has been observed 1153] that this action weakens with a decrease in the concentration of hydrogen peroxide, and that in solutions with a content below 40 wt. % hydrogen peroxide zinc already acts as a decomposition catalyst. This catalytic action has also been found in mixtures with other catalysts. Weiss 156] showed that metallic zinc decomposes hydrogen peroxide with evolution of hydrogen and oxygen. So far, no mechanism has been proposed to explain this dual action of zinc. The influence of cadmium has been studied only in weak solutions, and either weak catalytic properties are attributed to it, or it is considered completely ineffective.
After extraction, the anthrachiion solution contains about 0.1-0.3% water, small amounts of hydrogen peroxide (0.17 g/kg concentration is given as a typical concentration), as well as various oxidized organic substances, such as organic acids, aldehydes, ketones, etc. e. These compounds can poison the nickel hydrogenation catalyst and must therefore be removed prior to recirculation. According to the German process, the working solution is dried with an aqueous solution of potassium carbonate with a concentration of 33% (by weight), this solution also extracts part of the hydrogen peroxide. Organic matter and traces of water are removed by adsorption on the clay layer. Residual hydrogen peroxide is subjected to decomposition on a layer of nickel-silver catalyst, and sometimes a small amount (about 10%) of the reduced solution from the hydrogenator is added to the return liquid before it is fed to the carrier with a catalyst for better removal of hydrogen peroxide and dissolved oxygen. In this case, a small amount of water is formed, which remains in the working solution.
Hydrogen peroxide was used as a single-component fuel together with an aqueous solution of calcium or sodium permanganate as a catalyst. Such fuel was used for Focke-Wulf and Henkel aircraft with engine thrust of 300, 500 and 1000 kg and launchers for projectile aircraft. In these systems, the catalytic decomposition of hydrogen peroxide is carried out with the simultaneous supply of H2O2 and a concentrated solution of KaMnO2 to the LRE chamber. or Ca(MnO 4)2. The reaction starts quickly with a smooth rise in pressure up to 50-70 kg/cm2 in 0.01-0.02 sec.
Steam gas for driving the turbine is obtained either from a special component that is not a component of the engine fuel, or from components that the rocket engine runs on. Hydrogen peroxide is often used as a source of vapor gas. To obtain steam-gas from hydrogen peroxide, it is subjected to decomposition in a steam-gas generator with the help of catalysts - substances that promote decomposition.
Under the action of oxygen and moisture on many metals, small amounts of hydrogen peroxide are formed, which was determined qualitatively by a colorimetric method, for example, with a titanium salt, or by the Russell effect. This effect is based on the fact that photographic plates are very sensitive to very small amounts of hydrogen peroxide. Thus, Russell showed that a number of substances, including various metals, especially after fresh surface polishing, give photographic images when kept near a photographic plate in the dark. It has been proven that this is due to the release of hydrogen peroxide. Hydrogen peroxide by one of the indicated methods was found during the oxidation of the following metals zinc, lead, tin, silver, mercury, copper, aluminum, cadmium, magnesium and iron. It is likely that it is also formed during the oxidation of many other metals. It is very difficult to discover it on metals that are active catalysts for the decomposition of hydrogen peroxide, such as iron, copper and lead. Apparently, the concentration of hydrogen peroxide, which occurs during the autoxidation of metals, is determined by the relative rates of formation and decomposition reactions. The discovery of hydrogen peroxide by one or another author depends on the sensitivity of the technique used by him, as well as on the conditions of the experiment. Higher concentrations of hydrogen peroxide are found on the surfaces of freshly ground metal, and also (at least in the case of aluminum) in weakly or moderately acidic or slightly alkaline aqueous solutions. In the process of oxidation, the metal acquires a negative potential. The anodic polarization of the metal suppresses the formation of hydrogen peroxide, the cathodic polarization promotes this formation. It is not possible to say exactly whether the presence of both water and oxygen is required for the formation of hydrogen peroxide, but it is very likely that it is required. In one experiment, a sample of aluminum in dry nitrogen produced a faint photographic image, but it probably adsorbed oxygen and water (or only water) from the air prior to exposure to an inert atmosphere.
The ability of hydrogen peroxide to decompose in the presence of catalysts allows engines running on this oxidizer not to have a special ignition device for starting. On hydrogen peroxide, the so-called thermal start of the engine is possible. Hydrogen peroxide is fed into the prechamber (a small volume that communicates with the main combustion chamber), where it decomposes under the influence of a catalyst located here. Hot gaseous decomposition products of hydrogen peroxide enter the main combustion chamber of the engine. After the necessary pressure is created in the combustion chamber for normal combustion of the fuel, a combustible component is fed into it.
As polymerization catalysts, water-soluble peroxide compounds are most often used, which give free radicals during decomposition. Such compounds are hydrogen peroxide, potassium peroxide, persulfates and perborates. The peroxide compound soluble in the monomer is benzoyl peroxide. It was also found that diazoaminobenzene actively polymerizes butadiene. Tertiary amines soluble in at least one of the polymerizable components are used as reaction catalysts.
Formaldehyde Hydrogen peroxide Polymer P a 3 J Decomposition products Cadmium or zinc chelate compound in alkylidene diacetate medium, 10-80° C 10 w e same Catalyst
The catalyst can be used both in the form of an aqueous solution injected through a nozzle into the decomposition chamber simultaneously with hydrogen peroxide, and in solid form. In the latter case, a ceramic nozzle is impregnated with a catalyst, onto which sprayed hydrogen peroxide falls. 1 kg of solid catalyst can decompose up to 2000 kg of 80% hydrogen peroxide.
Hydrogen peroxide is a good oxidizing agent, especially in an alkaline solution. Excess peroxide is usually decomposed by boiling an alkaline solution. Decomposition is accelerated by the introduction of catalysts, such as salts of Nickel, iodide, platinum black. Shulek and Shchakach removed the excess oxidizing agent with chlorine water, and potassium cyanide was introduced to destroy the excess chlorine.
These methods are used to prepare porous elastomers and thermoplastics that are not affected by degradation products. A large number of pore-forming substances are used, of which the most common are sodium and ammonium bicarbonates, ammonium nitrate, calcium carbonate, diazo derivatives and diisocyanates. Gas-saturated activated carbon has been proposed as a pore-forming agent In the Telely process for the production of porous rubber, the gas source is hydrogen peroxide, which decomposes with the release of oxygen under the action of a yeast catalyst. evenly distributed throughout the plastic mass before gas evolution occurs.
Currently, there are several ways to obtain peracetic acid used for the epoxidation of various unsaturated compounds. The choice of method depends on the position in the molecule of the double bond being oxidized. There are two main epoxidation methods used in industry. First, hydrogen peroxide is added to a mixture of acetic acid, an unsaturated compound and an acid catalyst. The peracetic acid formed as an intermediate product oxidizes the olefin to a compound containing epoxy groups. In another method, acetaldehyde is oxidized with air in a suitable solvent to acetaldehyde monoperacetate, which upon thermal decomposition yields peracetic acid. Acetic acid and acetaldehyde formed as by-products are removed by distillation in vacuo. Because epoxidation converts peracetic acid to acetic acid, the process converts acetaldehyde to acetic acid as a by-product.
The relationship between homogeneous and heterogeneous catalysis has been studied only poorly, mainly because the elements capable of giving rise to both types of catalysis have not been studied over the entire range of variables (for example, pH and concentration) that determine the state of the catalyst. As a catalyst, in which one can observe the transition from a homogeneous mechanism to a heterogeneous one, one can name iron. In an acidic solution, the reaction is purely homogeneous. However, if the pH is increased, colloidal material begins to appear and at the same time there is a change in speed (see fig. 76 on page 440). At even higher pH, the formation of a macroscopic precipitate can be observed, as well as other kinetic changes. The rate of catalysis can also be affected by changes in the physical form (presence of a support for the catalyst, sintering of the catalyst, or a change in the crystal structure). Although the pH at which a colloidal substance begins to appear has not yet been fully determined, there is no doubt that the transition from homogeneous to heterogeneous decomposition with increasing pH is not subject to any doubt. However, there are still significant uncertainties about the nature of the mechanism change. In some cases, both types of decomposition can be qualitatively explained by the same mechanism, such as cyclic oxidation and reduction. At the same time, the formation of a complex or precipitation of a catalyst in a colloidal or solid state can determine the t-fraction of the total amount of catalyst present that is able to actually participate in the reaction and thus affect the observed rate of decomposition. Such a case of complex formation occurs in the catalysis of polymerization by the action of peroxides. In purely heterogeneous catalysis, the observed rate depends on the degree of dispersion of the solid catalyst, since this dispersion determines the size of the surface in contact with the medium. On the contrary, it is quite possible that in the transition from a homogeneous system to a heterogeneous one, the nature of the reaction to which hydrogen peroxide undergoes also changes radically, for example, the ionic mechanism can turn into a radical one. It is possible that as conditions change, there is a relatively fine gradation in the transition from one mechanism to another. When elucidating the differences between homogeneous and heterogeneous catalysis, one should always take into account the possible effect of adsorption from solution on homogeneous catalysis. Thus, monovalent silver, which does not have catalytic properties in homogeneous dispersion, is easily adsorbed by glass. In the adsorbed state, it can acquire catalytic properties as a result of either true reduction to metal, or only polarization. Subsequent use of the glass surface in contact with a more alkaline solution can also activate the adsorbed silver. This is especially noticeable in the case of the glass electrode surface.
The effect of these factors on metallic lead is very pronounced. If polished lead, devoid of an oxide film, is immersed in hydrogen peroxide, then its activity turns out to be very low. Gradually, a white precipitate is formed, which, after accumulation, turns into red lead, followed by a rapid manifestation of catalytic activity. If metallic lead is briefly immersed in a hydrogen peroxide solution and immediately removed, then a small amount of liquid adhering to the metal remains in a calm state for a short period of time, and then, after the formation of a film of red lead Pb304 on the metal, it sharply breaks away from the surface under the action of a violent decomposition. In this process, the dissolution of lead occurs, which is undoubtedly associated with the observed destruction of the passivity of lead under the action of hydrogen peroxide, however, peroxide does not affect the growth of dendrites on it. The practical application of lead catalysts for the decomposition of concentrated hydrogen peroxide in systems used for power generation is described.
It is often difficult to determine whether the peroxides isolated from the reaction mixture are hydrogen peroxide or whether they are organic peroxides, until very recently few attempts have been made to determine the structure of these peroxides. Conclusions regarding the nature of peroxides can be drawn on the basis of the following evidence: 1) the composition of the gas and liquid formed during the decomposition of peroxide (for example, hydrogen peroxide gives oxygen and water; hydroxyalkyl hydroperoxide, when decomposed with alkali, gives hydrogen and acid; methyl hydroperoxide, when decomposed by platinum black, gives carbon dioxide) 2) various color reactions, for example, reactions using titanium salt, which is considered very specific for hydrogen peroxide (see Chap. 10) 3) reaction characteristics with an acidic solution of potassium iodide (methyl hydroperoxide, for example, reacts only in the presence of sulphate ferrous iron as a catalyst, but does not react in the presence of ammonium molybdate, in addition, the rate of oxidation of iodide to iodine significantly depends on the nature of the peroxide) 4) the formation of insoluble inorganic peroxides, such as calcium peroxide or sodium peroxoborate, with the introduction of appropriate additives to the product, which proves the presence
In one particular case, when the presence of nitrate in a 30% (by weight) hydrogen peroxide solution turned out to be harmful, it was removed from it mainly by adsorption on activated carbon with relatively little decomposition of the peroxide. As a laboratory method, it is also proposed to purify hydrogen peroxide by rapidly adding, with stirring, first a solution of ferric chloride, and then calcium carbonate, and rapidly filtering the mixture through a Gooch crucible. Subsequent addition of concentrated sulfuric acid removes the residual yellow color and precipitates the calcium. The first two added substances probably form a precipitate of aqueous iron hydroxide (II), which, having a high adsorption capacity, can capture small amounts of impurities. However, iron compounds are powerful decomposition catalysts, and even small amounts remaining after said treatment can cause significant decomposition. It is difficult to imagine that this kind of technique, coupled with the introduction of unacceptable contamination, has any advantages over the method of precipitation with tin oxide hydrate. In the best case, a noticeable decomposition of the peroxide can occur; in the worst case, this process is associated with the danger associated with the addition of catalytically active substances to the peroxide, especially if they are introduced in a noticeable concentration. Therefore, the described method can by no means be recommended.
And siphons can be obtained from various companies producing hydrogen peroxide, and here we do not dwell on these issues. The most important precautions are to 1) avoid contact of the peroxide with active catalysts, such as materials containing iron, copper, manganese and most other metals, as well as dust and alkaline compounds that can cause rapid decomposition 2) avoid contact with organic substances that can ignite or form explosive mixtures with concentrated hydrogen peroxide 3) always ensure adequate ventilation of equipment in which hydrogen peroxide can be stored or temporarily 4) avoid excessively high temperatures. The physiological effects of hydrogen peroxide are described on page 153. Peroxide having a concentration of about 50 wt.% or less usually does not immediately ignite an accidentally spilled combustible material, such as clothing, but if it is allowed to dry, then, since water evaporates more easily, the concentration of peroxide increases, which sometimes leads to self-ignition. Contaminated materials containing catalytic impurities or other combustible substances, such as wood or clothing, especially wool, often ignite spontaneously when exposed to concentrated hydrogen peroxide. In all cases, spilled peroxide should be washed off with plenty of water.
In some industrial centers, one has to face the difficulty of eliminating wastewater containing hydrogen peroxide by dumping it into water bodies. Thus, concentrations of hydrogen peroxide exceeding 40 mg / l have a toxic effect on trout fry, lower concentrations are completely harmless over a 48-hour period. The best method for ridding the water of residual hydrogen peroxide depends on the nature of the other wastes contained in the water so, in the presence of reducing agents (hydrazine or methyl alcohol), for example in wastewater from a rocket test station, it is desirable to first cause an interaction between the peroxide and these substances. Since hydrogen peroxide easily decomposes in an alkaline environment, as well as under the action of various metal catalysts, according to one of the methods for treating residual peroxide, it is proposed to add lime to the water to bring the pH to 11, and then introduce a soluble manganese salt, such as chloride, so that the concentration of manganese is about 4 mg/l. At this pH manganese, apparently, turns into a fine precipitate of manganese oxide hydrate, which is a very effective catalyst. The mixture should be mixed until the peroxide is completely decomposed and, after the sediment has settled, the wastewater should be discharged into the reservoir. The settled sludge can probably be reused.
The principle of charge transfer is, of course, of great importance, but these phenomena are not yet quite clear and are not connected in a consistent reliable theory. For example, barium peroxide, almost all built from ions, is stable. The works cited below show that the introduction of electron-donating substituents into acyl peroxides accelerates decomposition. Apparently, any such comparisons are valid only for similar processes, i.e., for breaking the same bond in the same medium due to only a homogeneous or heterogeneous process involving the same or equivalent reagent, initiator and dicatalyst. Thus, if one considers homogenous decomposition in the gas phase, then organic peroxides are apparently less stable than hydrogen peroxide. On the contrary, the example of reactions with ferrous ion shows that hydrogen peroxide is the most reactive of all peroxides studied. In particular, one must distinguish between the sensitivity of any peroxide to explosion or detonation and the rate at which it reacts under strictly defined conditions. Fundamentals of General Chemistry Volume 2 Edition 3 (1973) -- [
And natural resources
Department of Chemistry and Ecology
STUDY OF THE REACTION RATE OF DECOMPOSITION
HYDROGEN PEROXIDE IN THE PRESENCE OF A CATALYST
GAS METHOD.
in the discipline "Physical and colloidal chemistry"
for the specialty 060301.65 − Pharmacy
Velikiy Novgorod
1 The purpose of the work……………………………………………………………………..3
2 Basic theoretical provisions…………………………………………….3
4 Experimental part………………………………………………………4
4.1 Decomposition of hydrogen peroxide in the presence of manganese dioxide MnO2 ………..…………………………………………………………………….4
4.2 Decomposition of hydrogen peroxide in the presence of a catalyst at temperature T2 .................................................................. ................................................. ................6
5 Requirements for the content of the report……………………………………………..6
6 Exemplary control questions and tasks……...……………………………7
1 OBJECTIVES OF THE WORK
1. Determine the rate constant, reaction order, half-life at temperature T1.
2. Construct a graph of the dependence of the amount of released O2 on time, determine graphically the half-life.
3. Determine the activation energy of the reaction, calculate the temperature coefficient of the reaction rate.
2 MAIN THEORETICAL PROVISIONS
The use of hydrogen peroxide in many technological processes, medicine and agriculture is based on its oxidizing properties. The process of H2O2 decomposition in aqueous solutions proceeds spontaneously and can be represented by the equation:
H2O2®H2O +1/2 O2
The process can be accelerated with a catalyst. These can be anions and cations, such as CuSO4 (homogeneous catalysis). Solid catalysts (coal, metals, salts, and metal oxides) also have an accelerating effect on the decomposition of H2O2. The course of a heterogeneous catalytic reaction of H2O2 decomposition is affected by the pH of the medium, the state of the surface, and catalytic poisons, for example, C2H5OH, CO, HCN, H2S.
In the cells of plants, animals, and humans, catalytic decomposition of hydrogen peroxide is also carried out. The process is carried out under the action of catalase and peroxidase enzymes, which, unlike non-biological catalysts, have exceptionally high catalytic activity and specificity of action.
The decomposition of H2O2 is accompanied by the release of O2. The volume of released oxygen is proportional to the amount of decomposed hydrogen peroxide. The paper uses the gasometric method.
3 SAFETY REQUIREMENTS
When performing this laboratory work, it is necessary to follow the general rules for working in a chemical laboratory.
4 EXPERIMENTAL
4.1 Decomposition of hydrogen peroxide in the presence of manganese dioxideMNO2 .
Before starting the experiment, it is necessary to prepare a catalyst: grease a small piece of a glass rod with BF glue or starch paste. It is necessary to grease only the end with glue, pour a little MnO2 powder on the watch glass, touch the end of the stick to the powder so that a small amount of MnO2 remains on the glass. Glue Dry for several minutes (1-2 min). The pressure inside the system for collecting H2O2 must be brought to atmospheric pressure: open the stopper of the reaction tube, use a balancing bottle to set the water level in the burette to zero.
The scheme of the device for measuring the rate of decomposition of H2O2 is shown in Fig. 1.
water
 |
test tube with H2O2
Gif" width="10">.gif" width="10"> catalyst
Fig.1 - Instrument for studying the kinetics of H2O2 decomposition.
2 ml of a 3% solution of H2O2 are measured with a pipette or measuring cylinder, poured into test tube 1. If the experiment is carried out at room temperature, prepare a stopwatch, a table for recording experimental data, Dip the catalyst applied on a piece of glass rod into the test tube. Close the reaction vessel with a stopper. The volume of oxygen released is recorded first after 30 s, then the interval can be increased to 1 min.
As the liquid level in the burette decreases, the equalizing bottle is lowered so that the liquid level in the burette and the bottle does not change, the level difference is minimal.
The reaction is considered complete when the liquid level in the buret stops falling.
The volume of oxygen corresponding to the complete decomposition of H2O2 -V¥ can be obtained if the reaction vessel is placed in a glass of hot water. After cooling the tube to room temperature. After that, the volume of O2 corresponding to the complete decomposition of H2O2 is determined.
Table - Experimental data
Assuming that the order of the reaction is first, the reaction rate constant is calculated from the first order kinetic equation:
Based on the results of the experiment, the average value of the reaction rate constant is calculated.
The half-life of hydrogen peroxide is calculated from the equation:
t0.5 = 0.693/k using the average rate constant.
Determine the rate constant and the half-life graphically, using the dependence Vt= f (t) and ln(V¥ - Vt) = f (t), which are shown in Fig. 2 and Fig. 3. Compare the results obtained by two methods - analytical and graphic.

V¥https://pandia.ru/text/80/128/images/image032_11.gif" width="211" height="12">.gif" width="616" height="64">
t, min t, min
Rice. 2 – Dependence Vt = f(t) Fig.3 – Dependence ln(V¥ – Vt) = f(t)
4.2 Decomposition of hydrogen peroxide in the presence of a catalyst at temperature T2
The experiment is repeated by placing the reaction vessel in a water bath or a glass of water at a temperature of T2 (as directed by the teacher). The data is entered into the table:
Knowing the rate constants k1 and k2 at two different temperatures, we can calculate the activation energy Ea using the Arrhenius equation:
Ea = 
In addition, you can calculate the temperature coefficient using the van't Hoff rule:
k2/k1 = γ ∆t/10
5 REQUIREMENTS FOR THE CONTENT OF THE REPORT
The report must contain:
1. purpose of the work;
2. results of measuring the volume of oxygen released during the decomposition of peroxide;
3. calculation of the reaction rate constant and the half-life (half-life) of hydrogen peroxide;
4. graph of dependence Vt = f(t) and the results of graphical determination of the half-life of hydrogen peroxide;
5. graph of dependence ln(V¥ – Vt) = f(t) to determine the reaction rate constant;
6. results of measurements of the volume of oxygen released during the decomposition of peroxide at an elevated temperature and calculation of the reaction rate constant;
7. calculation of the activation energy according to the Arrhenius equation and calculation of the temperature coefficient of the reaction rate according to the van't Hoff rule;
8. conclusions.
6 EXAMPLE QUESTIONS AND QUESTIONS
1. The reaction rate constant depends on:
a) the nature of the reagents;
b) temperature;
c) concentrations of reagents;
d) the time elapsed since the start of the reaction.
2. Order of reaction
a) formal value;
b) is determined only experimentally;
c) can be calculated theoretically;
d) is equal to the sum of the exponents p + q, in the equation υ = k · CAp · CBq.
3. Activation energy of a chemical reaction
a) the excess energy compared to the average energy of the molecules, necessary for the collision between the molecules to become active;
b) depends on the nature of the reagents;
c) measured in J/mol;
d) increases with the introduction of a catalyst into the system.
4. The half-life of a certain radioactive isotope is 30 days. Calculate the time after which the amount of the isotope will be 10% of the original.
5. The reaction of the first order at a certain temperature proceeds by 25% in 30 minutes. Calculate the half-life of the starting material.
6. How many times will the reaction rate increase with an increase in temperature by 40K, if the temperature coefficient of the reaction rate is 3?
7. With an increase in temperature by 40K, the rate of a certain reaction increased by 39.06 times. Determine the temperature coefficient of the reaction rate.
Hydrogen peroxide (peroxide) is a colorless syrupy liquid with a density that hardens at -. This is a very fragile substance that can decompose with an explosion into water and oxygen, and a large amount of heat is released:
Aqueous solutions of hydrogen peroxide are more stable; in a cool place they can be stored for quite a long time. Perhydrol - a solution that goes on sale - contains. It, as well as in highly concentrated solutions of hydrogen peroxide, contains stabilizing additives.
The decomposition of hydrogen peroxide is accelerated by catalysts. If, for example, a little manganese dioxide is thrown into a solution of hydrogen peroxide, then a violent reaction occurs and oxygen is released. Catalysts that promote the decomposition of hydrogen peroxide include copper, iron, manganese, as well as ions of these metals. Already traces of these metals can cause decay.
Hydrogen peroxide is formed as an intermediate product during the combustion of hydrogen, but due to the high temperature of the hydrogen flame, it immediately decomposes into water and oxygen.

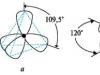
Rice. 108. Scheme of the structure of the molecule. The angle is close to , the angle is close to . Link lengths: .
However, if a hydrogen flame is directed at a piece of ice, traces of hydrogen peroxide can be found in the resulting water.
Hydrogen peroxide is also obtained by the action of atomic hydrogen on oxygen.
In industry, hydrogen peroxide is obtained mainly by electrochemical methods, for example, anodic oxidation of solutions of sulfuric acid or ammonium hydrosulfate, followed by hydrolysis of the resulting peroxysulfuric acid (see § 132). The processes taking place in this case can be represented by a diagram:
![]()
In hydrogen peroxide, hydrogen atoms are covalently bonded to oxygen atoms, between which a simple bond also occurs. The structure of hydrogen peroxide can be expressed by the following structural formula: H-O-O-H.
Molecules have significant polarity, which is a consequence of their spatial structure (Fig. 106).
In a hydrogen peroxide molecule, the bonds between hydrogen and oxygen atoms are polar (due to the displacement of common electrons towards oxygen). Therefore, in an aqueous solution, under the influence of polar water molecules, hydrogen peroxide can split off hydrogen ions, that is, it has acidic properties. Hydrogen peroxide is a very weak dibasic acid in an aqueous solution; it decomposes, albeit to a small extent, into ions:
Dissociation on the second stage
practically does not flow. It is suppressed by the presence of water - a substance that dissociates to form hydrogen ions to a greater extent than hydrogen peroxide. However, when hydrogen ions are bound (for example, when alkali is introduced into a solution), dissociation in the second stage occurs.
Hydrogen peroxide reacts directly with some bases to form salts.
So, under the action of hydrogen peroxide on an aqueous solution of barium hydroxide, a precipitate of the barium salt of hydrogen peroxide precipitates:
Salts of hydrogen peroxide are called peroxides or peroxides. They consist of positively charged metal ions and negatively charged ions, the electronic structure of which can be represented by the diagram:
![]()
The degree of oxidation of oxygen in hydrogen peroxide is -1, i.e., it has an intermediate value between the degree of oxidation of oxygen in water and in molecular oxygen (0). Therefore, hydrogen peroxide has the properties of both an oxidizing agent and a reducing agent, i.e., it exhibits redox duality. Nevertheless, oxidizing properties are more characteristic of it, since the standard potential of the electrochemical system
in which it acts as an oxidizing agent, is 1.776 V, while the standard potential of the electrochemical system
in which hydrogen peroxide is a reducing agent, is 0.682 V. In other words, hydrogen peroxide can oxidize substances that do not exceed 1.776 V, and restore only those that are more than 0.682 V. According to table. 18 (on page 277) you can see that the first group includes many more substances.
Examples of reactions in which it serves as an oxidizing agent are the oxidation of potassium nitrite
and isolation of iodine from potassium iodide:
It is used for bleaching fabrics and furs, used in medicine (3% solution - a disinfectant), in the food industry (for food preservation), in agriculture for dressing seeds, as well as in the production of a number of organic compounds, polymers, porous materials. As a strong oxidizing agent, hydrogen peroxide is used in rocket technology.
Hydrogen peroxide is also used to renew old oil paintings that have darkened over time due to the conversion of white lead into black lead sulfide under the influence of traces of hydrogen sulfide in the air. When such paintings are washed with hydrogen peroxide, lead sulfide is oxidized to white lead sulfate:

O.S.ZAYTSEV
EDUCATIONAL BOOK IN CHEMISTRY
FOR SECONDARY SCHOOL TEACHERS,
STUDENTS OF PEDAGOGICAL UNIVERSITIES AND SCHOOLCHILDREN OF GRADES 9–10,
DECIDED TO DEVOTE THEMSELVES TO CHEMISTRY AND NATURAL SCIENCE
TEXTBOOKTASK LABORATORY PRACTICESCIENTIFIC STORIES FOR READING
Continuation. See Nos. 4-14, 16-28, 30-34, 37-44, 47, 48/2002;
1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23,
24, 25-26, 27-28, 29, 30, 31, 32, 35, 36, 37, 39, 41, 42, 43, 44, 46, 47/2003;
1, 2, 3, 4, 5, 7, 11, 13, 14, 16, 17, 20, 22/2004
§ 8.1 Redox reactions
(continuation)
TASKS AND QUESTIONS
1. Using the electron-ion method of selecting stoichiometric coefficients, compose the equations of redox reactions that proceed according to the following schemes (the water formula is not indicated):


Please note that among the compounds there are organic substances! Try to find coefficients using oxidation states or valences.
2.
Choose any two equations of electrode reactions:

Compose one summary equation from the two written equations of electrode processes. Name the oxidizing agent and reducing agent. Calculate the emf of the reaction, its G and the equilibrium constant. Make a conclusion about the direction of the shift in the equilibrium of this reaction.
If you forgot what to do, remember what was said above. You write out any two equations from this list. Look at the values of their electrode potentials and rewrite one of the equations in the opposite direction. What, why and why? Remember that the numbers of given and received electrons must be equal, multiply the coefficients by a certain number (which?) and sum both equations. The electrode potentials are also summed up, but you do not multiply them by the number of electrons involved in the process. A positive EMF value indicates the possibility of a reaction. For calculation G and the equilibrium constants, substitute the EMF value you calculated into the formulas that were derived earlier.
3. Is an aqueous solution of potassium permanganate stable? In another way, the question can be formulated as follows: will the permanganate ion react with water to form oxygen if

4. Oxidation by air oxygen in an aqueous solution is described by the equation:
O 2 + 4H + + 4 e\u003d 2H 2 O, E= 0.82 V.
Determine whether it is possible to oxidize the substances written on the right side of any equation of task 2 with air oxygen. Reducing agents are written on the right side of these equations. The teacher will give you the equation number.
You may find this task difficult to complete. This is the main flaw of your character - it seems to you that the task is impossible, and you immediately give up trying to solve it, although you have all the necessary knowledge. In this case, you should write the reaction equation between oxygen and hydrogen ions and the equation of interest to you. See which of the reactions has a higher ability to donate electrons (its potential should be more negative or less positive), rewrite its equation in the opposite direction, changing the sign of the electrode potential to the opposite, and sum it with another equation. A positive EMF value will indicate that the reaction is possible.
5.
Write the equation for the reaction between permanganate ion and hydrogen peroxide H 2 O 2 . In the reaction, Mn 2+ and O 2 are formed. What odds did you get?
And I got the following equation:
7H 2 O 2 + 2 + 6H + = 2Mn 2+ + 6O 2 + 10H 2 O.
Find a mistake if I made one, or explain why your coefficients are different. This task is designed to test your ingenuity and knowledge of the material of other sections of chemistry.
The reaction of a permanganate ion with hydrogen peroxide in an acid solution (sulfuric acid) can be represented by several equations with different coefficients, for example:
5H 2 O 2 + 2 + 6H + = 2Mn 2+ + 5O 2 + 8H 2 O,
7H 2 O 2 + 2 + 6H + = 2Mn 2+ + 6O 2 + 10H 2 O,
9H 2 O 2 + 2 + 6H + = 2Mn 2+ + 7O 2 + 12H 2 O.
Indicate the reason for this and write at least one more equation for the reaction of permanganate ion with hydrogen peroxide.
If you managed to explain the reason for such a strange phenomenon, explain the reason for the possibility of writing the following equations:
3H 2 O 2 + 2 + 6H + = 2Mn 2+ + 4O 2 + 6H 2 O,
H 2 O 2 + 2 + 6H + = 2Mn 2+ + 3O 2 + 4H 2 O.
Can reactions proceed according to these two equations?
Answer. The reaction of permanganate ions with hydrogen peroxide is superimposed by a parallel decomposition reaction of hydrogen peroxide:
2H 2 O 2 \u003d O 2 + 2H 2 O.
You can sum the basic reaction equation with an infinite number of this equation and get a lot of equations with different stoichiometric coefficients.
6. This task can serve as the topic of an essay or report.
Discuss the possibility of passing the reduction reaction of Fe 3+ ions with hydrogen peroxide in an aqueous solution:
2Fe 3+ + H 2 O 2 \u003d 2Fe 2+ + O 2 + 2H +.
Calculate the emf of the reaction, its G and the equilibrium constant, using the standard electrode potentials:

The study of the dependence of the reaction rate on the concentration of the components showed that with an increase in the concentration of Fe 3+ or H 2 O 2 individually, the reaction rate doubles. What is the kinetic equation for the reaction? Determine how the reaction rate will change with an increase in the concentration of Fe 3+ or H 2 O 2 three times. Predict how the reaction rate will change when the solution is diluted two or ten times with water.
The following reaction mechanism has been proposed:
H 2 O 2 \u003d H + H + (fast),
Fe 3+ + H = Fe 2+ + HO 2 (slow),
Fe 3+ + HO 2 = Fe 2+ + H + + O 2 (fast).
Prove that this mechanism does not contradict the above dependence of the rate on the concentrations of reactants. What is the limiting stage? What is its molecularity and what is its order? What is the general order of the reaction? Pay attention to the existence of such complex ions and molecules as H and HO 2 , and to the fact that two or even three particles are formed in each reaction. (Why are there no stages with the formation of one particle?)
7. Translate into Russian.
An important reaction type is the electron-transfer reaction, also known as the oxidation-reduction, or redox, reaction. In such a reaction one or more electrons appear to be transferred from one atom to another. Oxidation is a word originally meant combination with oxygen gas, but so many other reactions were seen to resemble reactions with oxygen that the term was eventually broadened to refer to any reaction in which a substance or species loses electrons. Reduction is a gain electrons. The term seems to have its origins in metallurgical terminology: the reduction of an ore to its metal. Reduction is just the opposite of oxidation. An oxidation cannot take place without its having a reduction coupled with it; that is, electrons cannot be lost unless something else gains them.
LABORATORY RESEARCH
The tasks offered to you, as it was before, are short research papers. Reactions that are important not only in chemistry but also in ecology were selected for the experiments. It is not necessary to complete all the experiments - choose the ones that interest you. It is desirable to work in small groups (2-3 people each). This reduces the time of the experiment, avoids mistakes and, most importantly, allows you to participate in scientific communication, which develops scientific speech.
1. Redox properties of hydrogen peroxide.
Hydrogen peroxide H 2 O 2 is the most important oxidizing agent that is used in everyday life, in technology, in the purification of water from organic contaminants. Hydrogen peroxide is an environmentally friendly oxidizing agent, because its decomposition products - oxygen and water - do not pollute the environment. The role of hydrogen peroxide and peroxide organic compounds in the processes of biological oxidation-reduction is known.
3–6% solutions of hydrogen peroxide for domestic and educational purposes are usually prepared from a 30% solution by dilution with water. Hydrogen peroxide decomposes during storage with the release of oxygen (do not store in tightly closed containers!). The lower the concentration of hydrogen peroxide, the more stable it is. To slow down decomposition, additives of phosphoric, salicylic acids and other substances are used. The salts of iron, copper, manganese and the catalase enzyme have a particularly strong effect on hydrogen peroxide.
A 3% solution of hydrogen peroxide in medicine is used for washing the mouth and gargling with stomatitis and sore throat.
30% hydrogen peroxide solution is called perhydrol. Perhydrol is not explosive. Getting on the skin, perhydrol causes burns, burning, itching and blistering, while the skin turns white. The burnt area should be rinsed quickly with water. Perhydrol in medicine is used to treat purulent wounds and to treat gums with stomatitis. In cosmetology, it is used to remove age spots on the skin of the face. Hydrogen peroxide stains on clothing cannot be removed. Hydrogen peroxide is used in the textile industry to bleach wool and silk, as well as furs.
The production of concentrated (90–98%) hydrogen peroxide solutions is constantly growing. Store such solutions in aluminum vessels with the addition of sodium pyrophosphate Na 4 P 2 O 7 . Concentrated solutions may decompose explosively. A concentrated solution of hydrogen peroxide on an oxide catalyst decomposes at 700 °C into water vapor and oxygen, which serves as an oxidizer for fuel in jet engines.
Hydrogen peroxide can exhibit both oxidizing and reducing properties.
The role of an oxidizing agent for hydrogen peroxide is more typical:
H 2 O 2 + 2H + + 2 e\u003d 2H 2 O,
for example in react:
2KI + H 2 O 2 + H 2 SO 4 \u003d I 2 + K 2 SO 4 + 2H 2 O.
Hydrogen peroxide as a reducing agent:
1) in an acid environment:
H 2 O 2 - 2 e\u003d O 2 + 2H +;
2) in the basic (alkaline) medium:
H 2 O 2 + 2OH - - 2 e\u003d O 2 + 2H 2 O.
Reaction examples:
1) in an acid environment:
2KMnO 4 + 5H 2 O 2 + 3H 2 SO 4 = K 2 SO 4 + 2MnSO 4 + 5O 2 + 8H 2 O;
2) in the main environment:
2KMnO 4 + H 2 O 2 + 2KOH \u003d 2K 2 MnO 4 + O 2 + 2H 2 O
The oxidizing properties of hydrogen peroxide are more pronounced in an acidic environment, while the reducing properties are more pronounced in an alkaline one.
1a. Decomposition of hydrogen peroxide.
Pour 2–3 ml of hydrogen peroxide solution into a test tube and heat the solution in a water bath. Gas release should begin. (What?) Prove experimentally that this is exactly the gas that you expected to receive.
Drop a grain of manganese dioxide into another test tube with a solution of hydrogen peroxide. Prove that the same gas is released.
Write the equation for the decomposition of hydrogen peroxide and separately the equations for receiving and returning electrons. What type of redox reaction is this?
Calculate the EMF of the reaction if:
![]()
Which of these two reactions has the greater ability to donate electrons and should be rewritten in the opposite direction? From the value of the EMF of the reaction, calculate G reactions and the equilibrium constant.
Compare results with G and equilibrium constant obtained from thermodynamic data:
Did your calculations match? If there is some discrepancy in the results, try to find the reasons.
1b. Detection of hydrogen peroxide.
To a diluted and acidified with sulfuric acid solution (2-3 ml) of potassium iodide, add a few drops of hydrogen peroxide solution. The solution will turn yellow-brown. When a few drops of starch solution are added to it, the color of the mixture instantly turns blue. Write the reaction equation (formed substances you know!).
Calculate the EMF of the reaction to make sure that the reaction is possible (select the reaction you need):

1c. Black lead sulfide and hydrogen peroxide.
The old masters painted their paintings with paints prepared on the basis of lead white, which included the white basic carbonate 2PbCO 3 Pb(OH) 2 . Over time, lead white turns black, and paints based on them change their color due to the action of hydrogen sulfide, and black lead sulfide PbS is formed. If the painting is carefully wiped with a dilute hydrogen peroxide solution, the lead sulfide turns into white lead sulfate PbSO 4 and the painting almost completely returns to its original appearance.
Pour 1–2 ml of a 0.1M solution of lead nitrate Pb (NO 3) 2 or lead acetate Pb (CH 3 COO) 2 into a test tube (sold in a pharmacy as a lead lotion). Add some hydrogen sulfide or sodium sulfide solution. Drain the solution from the resulting black precipitate and act on it with a solution of hydrogen peroxide. Write reaction equations.
All lead compounds are poisonous!
1g Preparation of a solution of hydrogen peroxide from hydroperite.
If you were unable to get a solution of hydrogen peroxide, then for laboratory work you can use hydroperite, the tablets of which can be bought at a pharmacy.
Hydroperite is a complex compound of hydrogen peroxide with carbamide (urea) NH 2 CONH 2 H 2 O 2 . When dissolved in water, a solution of hydrogen peroxide and carbamide NH 2 CONH 2 is obtained. A solution of hydroperite is used instead of a solution of hydrogen peroxide as an antiseptic and for dyeing hair. To rinse the mouth and throat, dissolve 1 tablet in a glass of water (0.25% hydrogen peroxide solution). One tablet of hydroperite weighs 1.5 g and corresponds to 15 ml
(1 tablespoon) 3% hydrogen peroxide solution.
Calculate how many hydroperite tablets should be dissolved in 100 ml of water to obtain approximately 1% hydrogen peroxide solution. What volume of oxygen (N.O.) can be obtained from one tablet of hydroperite?
Empirically determine how many milliliters of oxygen can be obtained from one tablet of hydroperite. Propose the design of the device and assemble it. Bring the volume of released oxygen to normal conditions. To get more accurate calculation results, you can take into account the vapor pressure of water over the solution, which at room temperature (20 ° C) is approximately 2300 Pa.
Purpose and objectives 1. Purpose: To find out which products contain catalysts that accelerate the decomposition of hydrogen peroxide, and which do not. 2. Tasks: o Find out what a catalyst is o Conduct an experiment with hydrogen peroxide and find out which products are a catalyst. 1. Purpose: To find out which products contain catalysts that accelerate the decomposition of hydrogen peroxide, and which do not. 2. Tasks: o Find out what a catalyst is o Conduct an experiment with hydrogen peroxide and find out which products are a catalyst.


What products are catalysts? 1. We took a hematogen, dripped hydrogen peroxide and saw that oxygen is released, therefore. hydrogen peroxide decomposes. 2. We also took other foods, such as raw meat, raw potatoes, beets, bread, garlic, banana, cocoa, and found that they also contain a catalyst.


Conclusion In the course of the work, we found out that the products containing catalysts for the decomposition of hydrogen peroxide are: hematogen, raw meat, raw potatoes, beets, bread, garlic, banana, cocoa. They are not: apple, tea leaves, cookies, orange / tangerine, sausage, smoked meat, ketchup, honey, chocolate candy. We also learned what a catalyst is and how to conduct this experiment.