Configuration is the relative spatial arrangement of atoms or atomic groups in a macromolecule, which is set during the synthesis process and cannot be changed without breaking the chemical bonds of the main chain.
There are three types of configurational isomerism: local isomerism, cis-trans isomerism and stereoisomerism.
Local isomerism is characteristic of polymers with an asymmetric repeating unit (vinyl and vinylidene polymers, (methacrylates, etc.). So, for a vinyl monomer molecule
the substituents at the C atoms (1) (head) and (2) (tail) differ, and, therefore, three types of addition are possible (in a dyad, i.e., in two consecutive monomer units):

Head-to-head attachment is less likely than head-to-tail attachment, primarily due to steric hindrance. So, for example, in polyvinylidene fluoride (-CH 2 -CF 2 -) " and polymethyl methacrylate, the proportion of units attached according to the "head - head" type does not exceed 5-6%.
It is also possible to attach monomers according to the “tail - tail” type, however, this type of isomerism can be distinguished only for dyads of repeating units, and in the macromolecule the difference between the “tail - tail” and “head - head” attachments is leveled.
Cis-trans isomerism characteristic of polymers containing double bonds in the main chain (polydienes, polyacetylenes), and consists in the possibility of arranging substituents one at a time (cis isomer) or on opposite sides (trance- isomer) of the double bond plane:

stereoisomerism pronounced for synthetic polymers having asymmetric carbon atoms in the main chain, as well as for a wide range of natural polymers such as proteins, polysaccharides and nucleic acids.
In this case, two options are possible:
- 1) macromolecules contain in the main chain true asymmetric carbon atom and exhibit optical activity (polypropylene oxide, natural polymers);
- 2) macromolecules with pseudo-asymmetric carbon atom that do not exhibit optical activity.
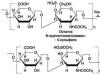
In biopolymers, asymmetric carbon atoms (marked?) are included in the molecules of the initial monomeric compounds - amino acids, carbohydrates (ribose, glucose, etc.):

and remain in each link of macromolecules after their synthesis, as, for example, in polypeptides (poly-/_-alanine) and polysaccharides (amylose): 
poly-1,4-a, D-glucopyranoside (amylose)
As a result, biopolymers have high optical activity. In the class of synthetic polymers, stereoisomerism is primarily characteristic of carbon chain vinyl and vinylidene polymers, the structure of which is shown schematically below.

In this case, the observed isomerism is due to the difference in the configuration of the tetrahedral carbon atom containing a non-hydrogen substituent X or substituents X and Z.
Strictly speaking, these carbon atoms are asymmetric because they are associated with four different groups (X, H or X, Z) and two chain segments that differ in length and end groups. However, these polymers do not exhibit optical properties due to the asymmetry of the nearest environment of carbon atoms, since identical CH 2 -CHX or CH 2 -CXZ groups adjoin the asymmetric carbon atom on both sides, and therefore these atoms are called pseudo-asymmetric. The regularity and nature of the arrangement of such centers of stereoisomerism is described by the concept "tact". Let us consider this type of isomerism in more detail using a vinyl polymer as an example.


Being maximally straightened without violating bond angles, the skeletal chain of such a carbon-chain polymer takes the form of a flat zigzag and can be placed in the pattern plane. In this case, the substituents at the carbon atom, the bonds of which are indicated by thick lines, are directed towards the reader, and the substituents, the bonds of which are indicated by thin lines, are directed away from the reader.
Let us apply the simplified method proposed in 1891 by the German organic chemist E. Fischer for determining and displaying stereoisomers. Let us project the polymer chain shown above onto a plane perpendicular to the plane of the sheet. As a result, we obtain a Fisher projection, for which all substituents X other than hydrogen are located on one side of the plane perpendicular to the sheet. This stereoisomer is called isotactic.

Another variant of the arrangement of substituents X is also obvious, namely, the strict alternation of substituents X on different sides of the plane. This stereoisomer is called syndiotactic.

In other words, isotactic polymer is a polymer, each monomer unit of which contains one center of stereoisomerism and the configuration of these centers is the same, and syndiotactic polymer - it is a polymer, each monomer unit of which contains one center of stereoisomerism and neighboring units have opposite configurations. If the location of the substituent X is random, then there is no stereoregularity, and such a configurational isomer is denoted as atactic.
The given data refer to polymers for which there is one iseudo-asymmetric carbon atom in the repeating unit. Note that such macromolecules are called monotactic. At didactic In polymers, the repeating unit contains two pseudo-asymmetric atoms.
Diisotactic polymers are obtained on the basis of 1,2-disubstituted alkenes of general structure (CHR=CHR"). In this case, the structure of the polymer product depends not only on the alternation L- and D-isomers in the monomer molecule, but also on its geometric isomerism. For example, for the 14 mg:-isomer, an ermtro-diisotactic polymer is formed:

Disyndiotactic polymers also form two syndiotactic structures ( erythro- and treo-), for which the structure of the main chain is identical.

Synthetic polymers are known that include truly asymmetric carbon atoms and, as a result, have optical activity. A typical representative of such compounds is polypropylene oxide, the Fisher projection of which is presented below (asymmetric carbon atoms are indicated by *).

Other examples of optically active polymers are polyamide based on (+)-2,2"-diaminobinaphthyl-1,G and terephthaloyl chloride

and also a polyamide obtained by the polycondensation of f-lysine and adipic acid dichloride in the presence of copper ions: 
Synthetic optically active polymers are obtained:
- 1) inactive polymer, leading to the introduction of optically active groups into its side substituents or to the creation of asymmetric centers by asymmetric synthesis;
- 2) polymerization or polycondensation optically active monomers, which occurs under conditions precluding racemization;
- 3) polymer-analogous transformations optically active polymers;
- 4)stereoselective polymerization one of the two optical isomers contained in the racemic mixture of the monomer;
- 5) asymmetric synthesis - stereospecific polymerization or polyaddition of symmetrical monomers.
A complex configuration composition is typical for diene polymers. During the polymerization of symmetrical butadiene, addition is possible due to the opening of 1,2- bonds or the simultaneous opening of 1,2- and 3,4- bonds (1,4-addition). The result is a mixture of two different polymer products: 1,4-polybutadiene and 1,2-polybutadiene:

For the first, m,is-trans configuration isomerism is possible, and for the second, local isomerism and stereoisomerism.
The situation becomes more complicated during the polymerization of unsymmetrical dienes (for example, isoprene), for which 1,4-, 1,2-, and 3,4-addition is observed:

In any variant of polymerization, the formation of local isomers occurs. Similarly to the case considered above, 1,4-polyisoprene is additionally characterized by r^r/c-oprais-isomerism, and 1,2- and 3,4-polyisoprene - by stereoisomerism.
The formation of a given configuration in the process of polymer synthesis, as well as the study of the configurational composition of macromolecules, is one of the most important problems in the synthetic and physical chemistry of polymers. The structure of polymers as a whole and their physical and mechanical properties are closely related to the configuration. Stereoregular polymers, as a rule, easily crystallize, while atactic polymers can exist only in the amorphous phase state. For example, isotactic polyvinyl chloride is a crystalline polymer with a melting point of 240°C, atactic polyvinyl chloride is an amorphous polymer with a glass transition temperature of 90°C. The glass transition temperature of isotactic polymethyl methacrylate is 40°C, and that of syndiotactic is 160°C. Natural rubber (1,4-gshs-polyisoprene) is a soft and pliable material with a glass transition temperature of minus 73°C, gutta-percha
(1,4-7iryans-polyisoprene) is a crystalline polymer with a melting point of 43°C.
Optically active polymers have higher mechanical properties, higher heat resistance than racemic products; they are suitable for the manufacture of glasses and films capable of rotating the plane of polarization of transmitted light (optical devices and light filters). The most important field of application of optically active polymers is the separation of optical isomers by chromatographic methods and their use as catalysts in asymmetric organic synthesis and as a matrix in asymmetric synthesis of polymers.
The local head-to-tail and head-to-head configurations are determined using the nuclear magnetic resonance (NMR) method. The characteristics of the signal of side substituent atoms identified by this method (1H, 13C, 15N, 19F) associated with the interaction of the spins of these nuclei depend on their mutual distance along the polymer chain, which makes it possible to estimate the proportion of head-to-tail additions. The same principle underlies the definition of stereoisomerism of macromolecules: in the isotactic configuration, the side groups are at a smaller distance from each other than in the syndiotactic one. Using the high-resolution NMR method, which identifies side groups, it is possible to fix signals from monomeric units that form iso-, syndio-, and heterotriads and calculate the proportion of these triads and their distribution in polymer chains.
Biological consequences of lipid peroxidation
Increased formation of free radicals in the body and the associated increase in lipid peroxidation processes (sometimes called "oxidative stress") is accompanied by a number of disturbances in the properties of biological membranes and cell functioning. Either protein structures or the lipid bilayer as a whole are damaged. Consequences of lipid peroxidation. The damaging effect of chain lipid oxidation on biological membranes is caused by the oxidation of thiol groups of proteins, an increase in the ionic permeability of membranes, and a decrease in the electrical strength of the lipid layer of membranes, which leads to "self-breakdown" of membranes by an electric field. A living cell has developed a whole system of protection against damage by free radicals. First, lipid peroxidation is accompanied by the oxidation of thiol (sulfhydryl) groups of membrane proteins (Pr).
Pr-SH + R -> RH + Pr-S
Pr-S + O 2 -> Pr-SO 2 -> molecular derivatives
The oxidation of proteins associated with lipid peroxidation and the formation of protein aggregates in the lens of the eye ends with its clouding; this process plays an important role in the development of senile and other types of cataracts in humans. An important role in cell pathology is also played by the inactivation of ion-transport enzymes, the active center of which includes thiol groups, primarily Ca2+-ATPase, which leads to an increase in the intracellular concentration of calcium ions and damage to the cell. The second result of lipid peroxidation is related to the fact that peroxidation products have the ability to directly increase the ion permeability of the lipid bilayer. Thus, it was shown that the products of lipid peroxidation make the lipid phase of membranes permeable to hydrogen and calcium ions. This leads to the fact that oxidation and phosphorylation in mitochondria are uncoupled, and the cell finds itself in conditions of energy starvation (i.e. lack of ATP). At the same time, calcium ions enter the cytoplasm, which damage cellular structures. The third (and perhaps most important) result of peroxidation is a decrease in the stability of the lipid layer, which can lead to electrical breakdown of the membrane by its own membrane potential, i.e. under the action of the difference in electrical potentials existing on the membranes of a living cell. Electrical breakdown leads to the complete loss of the membrane of its barrier functions.
The huge variety of functions of macromolecules in a cell is determined by their spatial organization. Therefore, one of the most important tasks of molecular biophysics is to elucidate the physical foundations for the formation of spatial structure and biological specificity. This refers to the fact that biological activity is sensitive to changes in the spatial structure of macromolecules.
Currently, several levels are purely conventionally distinguished - primary, secondary, tertiary and quaternary.
Primary structure of macromolecules- a sequence of links in the biopolymer chain linked to each other by strong covalent bonds. In proteins, this is the sequence of amino acids, and in NK, the sequence of nucleotides. Chains in polymers are formed by strong covalent bonds.
secondary structure is local, i.e. local ordering of individual sections of biomacromolecules (ordered structure of the main chain of a biopolymer).
Under the tertiary structure understand the spatial layout of the whole. Quaternary structure- it is understood as the spatial arrangement of several compactly organized polymer chains, chains with the formation of a supramolecular complex.
So what is meant by conformation? Conformation of a macromolecule- this is a way of laying a polymer chain (without breaking covalent bonds) due to the formation of a large number of weak bonds, as a result of which the thermodynamic most favorable and stable spatial structure of the macromolecule is formed. Changes in environmental parameters (temperature, pH, ionic strength, the action of denaturing factors) cause a conformational rearrangement of biomacromolecules with the formation of a new stable spatial structure.
All types of interactions between atoms, regardless of their physical nature, during the formation of various macromolecular bonds can be divided into 2 main types:
1. short-range interactions between atoms of neighboring units (covalent bonds);
2. long-range interactions between atoms, which, although they are far apart along the chain, but accidentally met in space as a result of chain bends (weak interactions - van der Waals forces, hydrophobic forces, electrostatic interactions and hydrogen bonds).
Introduction
Polymer molecules are a broad class of compounds, main the distinctive characteristics of which are a large molecular weight and high conformational flexibility of the chain. It can be said with confidence that all the characteristic properties of such molecules, as well as the possibilities of their application associated with these properties, are due to the above features.
Therefore, it is of great interest to study the possibility of a priori prediction of the chemical and physical behavior of a polymer based on an analysis of its structure. Such an opportunity is provided by the methods of molecular mechanics and molecular dynamics, implemented in the form of computer calculation programs.
Using these methods, the theoretical calculation of the most probable conformation of some oligomers with the number of monomeric units from 50 to 100 was carried out. Data were obtained that made it possible to determine the most probable conformation of molecules, the size of the Kuhn segment, and the number of monomeric residues in the segment.
Literature review
I. Polymers. Features of the structure and properties.
Polymers are high-molecular substances, the molecules of which consist of repeating structural elements - links connected in chains by chemical bonds, in an amount sufficient for the occurrence of specific properties. The following abilities should be attributed to specific properties:
1. the ability to significant mechanical reversible highly elastic deformations;
2. to the formation of anisotropic structures;
3. to the formation of highly viscous solutions when interacting with a solvent;
4. to a sharp change in properties when adding negligible additives of low molecular weight substances.
The given physicochemical features can be explained on the basis of the understanding of the structure of polymers. Speaking about the structure, one should imply the elemental composition of the substance, the order of the bonds of atoms, the nature of the bonds, the presence of intermolecular interactions. Characteristic of polymers is the presence of long chain molecules with a sharp difference in the nature of bonds along the chain and between chains. Of particular note is that there are no isolated chain molecules. The polymer molecule is always in interaction with the environment, which can have both a polymeric character (the case of a pure polymer) and the character of an ordinary liquid (diluted polymer solutions). Therefore, to characterize a polymer, it is not enough to indicate the type of bonds along the chain - it is also necessary to have information about the nature of intermolecular interaction. It should be borne in mind that the characteristic properties of polymers can only be realized when the bonds along the chain are much stronger than the cross-links formed due to intermolecular interactions of any origin. This is precisely the main feature of the structure of polymer bodies. Therefore, it can be argued that the entire complex of anomalous properties of polymers is determined by the presence of linear chain molecules with a relatively weak intermolecular interaction. The branching of these molecules or their connection into a network introduces some changes in the complex of properties, but does not change the state of affairs in essence as long as sufficiently long chain linear segments remain. On the contrary, the loss of the chain structure of molecules during the formation of globules or dense networks from them leads to the complete loss of the entire complex of properties characteristic of polymers.
The consequence of the above is the appearance of flexibility of the chain molecule. It lies in its ability to change shape under the influence of the thermal motion of the links or the external field in which the polymer is placed. This property is associated with the internal rotation of individual parts of the molecule relative to each other. In real polymer molecules, the bond angles have a well-defined value, and the links are not randomly located, and the position of each subsequent link turns out to be dependent on the position of the previous one.
Polymers that exhibit sufficiently intense torsional vibrations are called flex chain, and polymers in which the rotations of one part of the chain relative to the other are difficult - rigid chain.
This means that molecules can rotate and change their structure without breaking chemical bonds, forming various conformations, which are understood as various spatial forms of a molecule that arise when the relative orientation of its individual parts changes as a result of internal rotation of atoms or groups of atoms around simple bonds, bond bending, etc. .
II. Conformational analysis of polymers.
Conformational analysis is a section of stereochemistry that studies the conformations of molecules, their interconversions, and the dependence of physical and chemical properties on conformational characteristics. Each specific conformation corresponds to a specific energy. Under normal conditions, the molecule tends to move from the energetically least advantageous position to the most advantageous one. The energy required to move a molecule from a position with a minimum value of potential energy to a position corresponding to its maximum value is called potential barrier to rotation. If the level of this energy is high, then it is quite possible to isolate molecules with a certain spatial structure. The set of conformations that are in the vicinity of the energy minimum with an energy below the corresponding potential barrier is a conformer. The change in the conformation of the macromolecule occurs due to the limitation of the rotation of units around the bonds, as a result of which it usually takes the most probable form of a random coil. Various intra- and intermolecular interactions can lead to ordered conformations, as well as to an extremely folded globular conformation. Of exceptional importance is the conformational analysis in biochemistry. The chemical and biological properties of biopolymers depend to a large extent on their conformational properties. Conformational changes are an essential part of almost all biochemical processes. For example, in enzymatic reactions, the recognition of a substrate by an enzyme is determined by the spatial structure and the possibilities of mutual conformational adjustment of the participating molecules.
The following conformations are known:
The conformation of the macromolecular coil, i.e. more or less folded conformation, which the coil can take under the influence of thermal motion;
The conformation of an elongated rigid stick (or rod);
The helix conformation characteristic of proteins and nucleic acids also occurs in vinyl polymers and polyolefins, but they are not stabilized by hydrogen bonds and therefore less stable. The spiral can be either left-handed or right-handed, because it does not affect strength.
Globule conformation, i.e. very compact spherical particle;
Folded conformation, characteristic of many crystalline polymers;
“Crankshaft” or “Crank” Conformation
Each conformation of a macromolecule has a certain size. The theoretical calculation of the size of macromolecules was first made for a freely articulated chain, which, under the influence of thermal motion, can coil into a ball. The distance between the ends of such a macromolecular coil is denoted by h or r. Obviously, it can vary from 0 to L (the length of a fully unfolded chain). To calculate intermediate values of h, the apparatus of statistical physics (methods of molecular mechanics) is used, since there are a very large number of links in one chain.
A similar calculation can be made for a chain with fixed bond angles, replacing it with a freely articulated chain (a chain in which the links do not interact). In a freely articulated chain, the position of each link does not depend on the position of the previous one. In a real chain, the positions of the links are interconnected. However, for a very large chain length between sufficiently distant links, the interaction is negligibly small. If such links are connected by lines, then the directions of these lines are independent. This means that a real chain consisting of n monomer units of length l can be divided into N independent statistical elements (segments, segments) of length A.
It is believed that a statistical element, or a chain segment, of length A, the position of which does not depend on the position of neighboring segments, is called thermodynamic segment or Kuhn segment.
The length of the maximum elongated chain without violation of bond angles is called contour chain length L. It is related to the segment length by the relation
III. Empirical chemical methods of calculation.
For theoretical prediction of the most probable conformation of a molecule, the method of molecular mechanics is used. Molecular mechanics is a computational empirical method for determining the geometric characteristics and energy of molecules. It is based on the assumption that the energy of a molecule can be represented by the sum of contributions that can be attributed to bond lengths, bond angles, and torsion angles. In addition, in the general expression for energy there is always a term that reflects the van der Waals interaction of valence-unbound atoms, and a term that takes into account the electrostatic interaction of atoms and determines the presence of effective atomic charges.
E \u003d E sv + E shaft + E torus + E vdv + E cool
To calculate the first two terms, Hooke's law known from mechanics is most often used:
E st \u003d S k r (r - r 0) 2
It is assumed that the most thermodynamically stable conformation corresponds to the minimum energy. The method of molecular mechanics makes it possible to obtain information for a complete description of the geometry of various conformers in the ground state.
1.3. Macromolecular configuration
The concept of configuration includes a certain spatial arrangement of atoms of macromolecules, which does not change during thermal motion. The transition from one configuration to another is impossible without breaking chemical bonds.
There are: 1) link configuration, 2) short-range order - the configuration of link attachment, 3) long-range order - the configuration of large sections (for example, blocks and their alternation, or the length and distribution of branches), 5) the configuration of an elongated chain as a whole.
Link configuration. Examples are the cis and trans configurations of diene polymers
1,4-cis-polyisoprene 1,4-trans-polyisoprene (natural rubber) (gutta-percha) Another example would be l,d-isomerism. For instance,
for polymers with ~CH2 –CHR~ units, where R is any radical, the formation of two isomers is possible: l is left-handed, and d is right-handed
Link attachment configuration(short order). The links in the chain can be connected in a head-to-tail and head-to-head fashion:

is head-to-tail attachment, and head-to-head attachment requires overcoming large activation barriers.
For copolymers, the types of structural isomers increase compared to homopolymers. For example, for copolymers of butadiene and styrene, it is possible:
1. sequential alternation of links -A-B-A-B-A-B-,
2. combination of links in the form of dyads and triads–AA–BBB–AA–BBB– ,
3. statistical combination of links–AA–B–AA–BBB–A–B– . Far configuration order spreads on
tens and hundreds of atoms in the main chain. For example, large sequences of blocks in block copolymers or large sequences of units with the same stereoregularity (for example, polymers with isotactic, atactic and syndiotactic structures).
Isotactic Atactic Syndiotactic
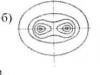
Overall circuit configuration is determined by the mutual arrangement of large sequences of links (with a long-range order). For example, for branched macromolecules, various types of configurations are shown in Fig. 4.
Rice. 4. Configurations of macromolecules

1.4. Conformation of macromolecules
A conformation is a variable distribution in space of atoms or groups of atoms that form a macromolecule. The transition from one conformation to another can occur due to rotation, rotation, or oscillation of links around single bonds under the action of thermal motion or external forces and is not accompanied by the breaking of chemical bonds.
Polymers can take various conformations:
Statistical tangle is a folded conformation. It is formed when the intensity of the internal thermal motion prevails over the external influence. Characteristic of linear polymers [PE, PP, PB, PIB and ladder polymers (polyphenylenesiloxane).
Helix - is formed in polymers due to H-bonds (for example, in protein molecules and nucleic acids).
A globule is a very compact particle close to spherical in shape. It is characteristic of polymers with strong intramolecular interaction (for example, in PTFE).
Rod or string found in alkyl polyisocyanates.
Fold conformation. It is characteristic of polymers in a crystalline state (for example, in PE).
Crankshaft Conformation realized in poly-n-benzamide.
Fig.5. Conformations of macromolecules
1.5. Flexibility of macromolecules
Flexibility is one of the most important characteristics of polymers, which determines the highly elastic, relaxation, and thermomechanical properties of polymers, as well as the properties of their solutions. Flexibility characterizes the ability of macromolecules to change their shape under the influence of thermal motion of links or external mechanical influences. Flexibility is due to the internal rotation of links or parts of macromolecules relative to each other. Consider the phenomenon of internal rotation in molecules on the example of the simplest organic compound - the ethane molecule.
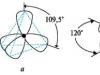
In the ethane molecule (CH3 -CH3) carbon atoms are bonded to hydrogen atoms and to each other by covalent (σ-bonds), and the angle between the directions of σ-bonds (valence angle) is 1090 28/. This causes a tetrahedral arrangement of substituents (hydrogen atoms) in space in the ethane molecule. Due to thermal motion in the ethane molecule, one CH3 group rotates relative to the other around the C-C axis. In this case, the spatial arrangement of atoms and the potential energy of the molecule are continuously changing. Graphically, various extreme arrangements of atoms in a molecule can be represented as projections of the molecule onto a horizontal plane (Fig. 6). Let us assume that in position a the potential energy of the molecule is U1, and in position b it is U2, while U1 ≠ U2, i.e. the positions of the molecule are energetically unequal. Position b, in which the H atoms are located one below the other, is energetically unfavorable, since repulsive forces appear between the H atoms, which tend to transfer the atoms to the energetically favorable position a. If accept
U1 =0, then U2 =max.

Rice. 6. Projection formulas for extreme arrangements of H atoms in space in an ethane molecule.
Rice. 7. Dependence of the potential energy of the molecule on the angle of rotation of the methyl group.
When one CH3 group is rotated relative to another by 600, the molecule goes from position a to b, and then after 600 again to position a, and so on. The change in the values of the potential energy of the ethane molecule from the angle of rotation φ is shown in Fig.7. Molecules with lesser symmetry (for example, the dichloroethane molecule) have a more complex dependence U=f(φ).
Potential (U 0 ) or activation barrier rotation
ion is the energy required for the transition of the molecule from the position of the minimum to the position of the maximum potential energy. For ethane, U0 is small (U0 = 11.7 kJ/mol) and at
At normal temperature, CH3 groups rotate around the C-C bond at high speed (1010 rpm).
If the molecule has an energy reserve less than U0, then there is no rotation and only oscillation of the atoms occurs relative to the position of the minimum energy - this is limited or
slow rotation.
In polymers, due to intra- and intermolecular interactions, the dependence U=f(φ) has a complex shape.
If one position of the chain link is characterized by potential energy U1, and the other - by U2, then the energy of transition from one position to another is equal to the difference ∆U= U1 - U2. The difference between the transition energies ∆U from one equilibrium position of a macromolecule unit to another characterizes thermodynamic flexibility. It determines the ability of the chain to bend under the influence of thermal motion.
Another characteristic of flexibility is the speed at which links move from one position to another. The rate of conformational transformations depends on the ratio of U0 and the energy of external influences. The more U0 , the slower the turns of the links and the less flexibility. The flexibility of macromolecules, determined by the value of U0, is called kinetic flexible
Factors that determine the flexibility of macromolecules
These factors include: the U0 value, polymer MM, density of the spatial network, size of substituents, and temperature.
Potential rotation barrier (U 0 ). The value of U0 depends on intra- and intermolecular interactions. Let us consider the factors affecting U0 and chain flexibility in carbon-chain polymers.
Carbochain polymers
In carbon chain polymers, saturated hydrocarbons are the least polar. Their intra- and intermolecular interactions are small, and the values of U0 and ∆U are also small, therefore, polymers have high kinetic and thermodynamic flexibility. Examples: PE, PP, PIB.
The values of U0 are especially low for polymers, in the chain of which there is a double bond next to the single bond.
–CH2 –CH=CH–CH2 – Polybutadiene
lar groups leads to intra- and intermolecular interactions. In this case, the degree of polarity significantly affects
With the introduction of polar groups, three cases are possible in terms of their effect on flexibility:
1. Polar groups are closely spaced and strong interactions are possible between them. The transition of such polymers from one spatial position to another requires overcoming large U0, so the chains of such polymers are the least flexible.
2. Polar groups are rarely located in the chain and there is no interaction between them. The values of U0 and ∆U are small and the polymers have high kinetic and thermodynamic flexibility.

-CF 2 -CF 2 -
Example: Polychloroprene
3.Polar groups are arranged so that the electric fields are mutually compensated. In this case, the total dipole moment of the macromolecule is equal to zero. Therefore, the values of U0 and ∆U are low, and polymers have high kinetic and thermodynamic flexibility.
Example: PTFE
Heterochain polymers
In heterochain polymers, rotation is possible around C–O, C–N, Si–O, and C–C bonds. The values of U0 for these bonds are small and the chains have sufficient kinetic flexibility. Examples: polyesters, polyamides, polyurethanes, silicone rubbers.
However, the flexibility of heterochain polymers can be limited by intermolecular interactions due to the formation of H-bonds (for example, in cellulose, polyamides). Cellulose is one of the rigid chain polymers. It contains a large number of polar groups (–OH) and therefore intra- and intermolecular interactions and high values of U0 and low flexibility are characteristic of cellulose.
Molecular weight of the polymer. An increase in the molecular weight of the polymer increases chain folding and, therefore, long macromolecules
have greater kinetic flexibility compared to short macromolecules. As the MM increases, the number of conformations that a macromolecule can adopt increases and the flexibility of the chains increases.
Spatial mesh density. The more chemical bonds between macromolecules, the less chain flexibility, i.e. as the density of the spatial grid increases, the flexibility decreases. An example is the decrease in chain flexibility with an increase in the number of crosslinks in the resol series.< резитол<резит.
Effect of size and number of substituents. An increase in the number of polar and large substituents reduces the mobility of the macromolecule units and reduces the kinetic flexibility. An example is the decrease in the flexibility of butadiene-styrene copolymer macromolecules with an increase in the content of bulky phenyl substituents in the chain.
If there are two substituents at one carbon atom in the main chain of the polymer (for example, OCH3 and CH3 in PMMA units), then the macromolecule becomes kinetically rigid.
Temperature. As the temperature rises, the kinetic energy of the macromolecule increases. As long as the value of the kinetic energy is less than U0, the chains perform torsional vibrations. When the kinetic energy of the macromolecule becomes equal to or exceeds U0, the links begin to rotate. With an increase in temperature, the value of U0 changes little, while the speed of rotation of the links increases and the kinetic flexibility increases.
Control questions
1 General information about polymers, concepts, definitions.
2 Define and give examples of organic, non-
organic and organoelement polymers.
2 Classification of homochain polymers, examples.
3 Classification of heterochain polymers, examples.
4 Thermodynamic and kinetic flexibility of macromolecules. What factors affect the flexibility of macromolecules?
5 What is the configuration of macromolecules and what types of configurations of macromolecules are possible? Examples.
6 What is the conformation of macromolecules and what types of conformations of macromolecules are possible? Examples.
7 What parameters characterize the molecular weight, molecular weight distribution and polydispersity of polymers?
8 Molecular characteristics of oligomers.
9 Fractionation of polymers and construction of molecular curves cular mass distribution.
macromolecule configuration otherwise primary structure(English) - spatial arrangement of atoms in . It is determined by the values of bond angles and the lengths of the corresponding bonds.
Description
The configuration of a macromolecule is determined by the mutual arrangement of its constituent monomer units, as well as by their structure. Currently, the term "structure" or "primary structure" is usually used to describe the configuration of macromolecules.
A distinction is made between short-range (configuration of attachment of neighboring units) and long-range configurational order, which characterizes the structure of sufficiently extended sections of macromolecules. A quantitative measure of tact (order) is the degree of stereoregularity. In addition, tacticity can be described by the number of different types of pairs of nearest neighbors (di-, tri-, tetrads), the distribution of which is determined experimentally. A quantitative characteristic of the configuration of statistical network macromolecules, for example, is the crosslink density, i.e., the average chain section between network nodes.
The configuration of macromolecules is determined by the methods of X-ray diffraction analysis, birefringence, etc. As a rule, each method is the most "sensitive" to any configuration characteristic; Thus, NMR in many cases makes it possible to quantitatively characterize the short-range configurational order in