The oxygen subgroup includes five elements: oxygen, sulfur, selenium, tellurium and polonium (a radioactive metal). These are the p-elements of the VI group of the periodic system of D.I. Mendeleev. They have a group name - chalcogens, which means "forming ores."
Properties of elements of the oxygen subgroup
|
Properties |
Those |
Ro |
|||
|
1. Order number |
|||||
|
2. Valence electrons |
2 s 2 2p 4 |
Z s 2 3r 4 |
4 s 2 4r 4 |
5s 2 5p 4 |
6s 2 6p 4 |
|
3. Energy Ionization of atom, eV |
13,62 |
10,36 |
9,75 |
9,01 |
8,43 |
|
4. Relative electronegativity |
3,50 |
2,48 |
2,01 |
1,76 |
|
|
5. The oxidation state in connections |
1, -2, |
2, +2, +4, +6 |
4, +6 |
4, +6 |
2, +2 |
|
6. Atomic radius, nm |
0,066 |
0,104 |
0,117 0,137 |
0,164 |
|
Chalcogen atoms have the same structure of the external energy level - ns 2 nr 4 . This explains the similarity of their chemical properties. All chalcogens in compounds with hydrogen and metals exhibit an oxidation state of -2, and in compounds with oxygen and other active non-metals, usually +4 and +6. For oxygen, as well as for fluorine, an oxidation state equal to the group number is not typical. It exhibits an oxidation state of usually -2 and in combination with fluorine +2. Such values of oxidation states follow from the electronic structure of chalcogens
The oxygen atom has two unpaired electrons in the 2p sublevel. Its electrons cannot be separated, since there is no d-sublevel at the outer (second) level, that is, there are no free orbitals. Therefore, the valency of oxygen is always equal to two, and the oxidation state is -2 and +2 (for example, in H 2 O and OF 2). These are the same valencies and oxidation states of the atom of sulfur in the unexcited state. Upon transition to an excited state (which takes place during the supply of energy, for example, during heating), at the sulfur atom, the 3 R— and then 3s electrons (shown by arrows). The number of unpaired electrons, and, consequently, the valency in the first case is four (for example, in SO 2), and in the second - six (for example, in SO 3). Obviously, even valencies 2, 4, 6 are characteristic of sulfur analogues - selenium, tellurium and polonium, and their oxidation states can be equal to -2, +2, +4 and +6.
Hydrogen compounds of elements of the oxygen subgroup are responsible formula H 2 R (R - element symbol): H 2 O, H 2 S , H 2 S e, H 2 Te. They callare hydrogen chalcides. When dissolved in water, they formacids. The strength of these acids increases with increasing atomic number of the element, which is explained by a decrease in energy bonds in the series of compounds H 2 R . Water dissociating into H + and O ions H - , is amphoteric electrolyte.
Sulfur, selenium and tellurium form the same forms of compounds with oxygen of the type R O 2 and R About 3- . They correspond to acids of the type H 2 R O 3 and H 2 R About 4- . With an increase in the ordinal number of the element, the strength of these acids decreases.vaet. All of them exhibit oxidizing properties, and acids of the type H 2 R About 3 are also restorative.
The properties of simple substances naturally change: with an increase incharge of the nucleus, non-metallic ones weaken and metallic ones increase. properties. So, oxygen and tellurium are non-metals, but the latter hasmetallic luster and conducts electricity.
Trans-argonoid sulfur compounds are more stable than the corresponding chlorine compounds, and phosphorus compounds are even more stable. Perchloric acid and perchlorates are strong oxidizing agents, while sulfuric acid and sulfates are weak oxidizing agents, and phosphoric acid and phosphates are even weaker. This difference in properties corresponds to the electronegativity values X= 3 for Cl, 2.5 for S, 2.1 for P, and Δх(relative to oxygen) is 0.5 for Cl, 1.0 for S, 1.4 for P. The characteristic heats of reaction given below reflect the increase in values Δх:
Hcl (g.) + 2O 2 (g.) → HclO 4 (l.) + 8 kJ mol -1
H 2 S (g.) + 2O 2 (g.) → H 2 SO 4 (l.) + 790 kJ mol -1
H 3 P (g.) + 2O 2 (g.) → H 3 RO 4 (l.) + 1250 kJ mol -1
The stable compounds of sulfur, selenium and tellurium correspond to several oxidation states from -2 to +6, as shown in the attached diagram:
6 SO 3 , H 2 SO 4 , SF 6 H 2 SeO 4 , SeF 6 TeO 3 , Te(OH) 6 , TeF 6
4 SO 2 , H 2 SO 3 SeO 2 , H 2 SeO 3 TeO 2
0 S 8 , S 2 Se Te
2 H 2 S, S 2- H 2 Se H 2 Te
Sulfur oxides
normal valent sulfur oxide(monoxide) SO is much less stable than the transargonoid oxides SO 2 and SO 3 . The heats of their formation have the following values:
1 / 8S 8 (c.) + 1 / 2O 2 (g.) → SO (g.) - 7 kJ mol -1
1 / 8S 8 (c.) + O 2 (g.) → SO 2 (g.) + 297 kJ mol -1
1/8S 8 (q.) + 3/2O 2 (g.) → SO 3 (g.) + 396 kJ mol -1
It follows from the first two equations that the decomposition of sulfur oxide into sulfur dioxide and sulfur is accompanied by the release of a large amount of heat
2SO (g.) → 1/8S 8 (c.) + SO 2 (g.) + 311 kJ mol -1
Therefore, it is not surprising that sulfur oxide is not known to be a stable compound, but exists only as extremely reactive molecules in a very rarefied gaseous state or in frozen matrices. This oxide has the structure
with two electrons having parallel spins, and resembles O 2 and S 2 molecules.
Sulfur dioxide (dioxide) SO 2 is formed during the combustion of sulfur or sulfides, such as pyrite (FeS 2)
S + O 2 → SO 2
FeS 2 + 11O 2 → 2Fe 2 O 3 + 8SO 2
It is a colorless gas with a characteristic pungent odor. The melting and boiling points of sulfur dioxide are -75 and -10 °C, respectively.
In the laboratory, sulfur dioxide is usually produced by the action of a strong acid on solid sodium hydrogen sulfite.
H 2 SO 4 + NaHSO 3 → NaHSO 4 + H 2 O + SO 2
It can be cleaned and dried by bubbling through concentrated sulfuric acid. Sulfur dioxide has the following electronic structure:
This structure uses one 3 d-orbital, as well as 3 s-orbital and three 3 p-orbitals. The experimentally established sulfur-oxygen bond length is 143 pm; this is somewhat less than the value of 149 pm that would be expected for a double bond. The O-S-O angle is 119.5°.
Large quantities of sulfur dioxide are used to produce sulfuric acid, sulfurous acid and sulfites. SO 2 kills fungi and bacteria and is used in the canning and drying of prunes, apricots and other fruits. A solution of acidic calcium sulfite Ca(HSO 3) 2 obtained by the reaction of sulfur dioxide with calcium hydroxide is used in the production of paper pulp from wood. It dissolves lignin, the substance that holds cellulose fibers together, and releases these fibers, which are then processed into paper.
Trioxide (trioxide) sulfur SO 3 is formed in very small quantities during the combustion of sulfur in air. It is usually produced by the oxidation of sulfur dioxide with air in the presence of a catalyst. The formation of this compound from simple substances is exothermic, but less exothermic (per oxygen atom) than the formation of sulfur dioxide. Feature of balance
SO 2 (g) + 1/2O 2 (g) → SO 3 (g)
lies in the fact that a satisfactory yield of SO 3 can be obtained at low temperatures; the reaction proceeds almost completely. However, at low temperatures, the reaction rate is so slow that the direct combination of reactants cannot be the basis of an industrial process. At high temperatures, when a satisfactory reaction rate is reached, the yield is low due to the unfavorable equilibrium position.
The solution to this problem was the discovery of appropriate catalysts (platinum, vanadium pentoxide), which accelerate the reaction without affecting its equilibrium. The catalytic reaction does not take place in the gas mixture, but on the surface of the catalyst when molecules come into contact with it. In practice, sulfur dioxide obtained by burning sulfur or pyrite is mixed with air and passed over a catalyst at a temperature of 400-450°C. Under these conditions, approximately 99% of sulfur dioxide is converted to sulfur trioxide. This method is mainly used in the production of sulfuric acid.
Sulfur trioxide is a highly corrosive gas; it combines vigorously with water to give sulfuric acid
SO 3 (g.) + H 2 O (l.) → H 2 SO 4 (l.) + 130 kJ mol -1
Rice. 8.3. Sulfur trioxide and some oxyacids of sulfur.
Sulfur trioxide readily dissolves in sulfuric acid to form oleum, or fuming sulfuric acid, consisting mainly of disulfuric acid H 2 S 2 O 7 (also called pyrosulfuric acid)
SO 3 + H 2 SO 4 ⇔ H 2 S 2 O 7
At 44.5°C, sulfur trioxide condenses into a colorless liquid, which solidifies at 16.8°C to form transparent crystals. This substance is polymorphic, and the crystals formed at 16.8°C are an unstable form (α-form). The stable form is silky asbestos-like crystals that form when alpha crystals or liquid are kept for a short time in the presence of traces of moisture (Fig. 8.3). There are also several other forms of this substance, but they are difficult to study due to the extremely slow transformation of one form into another. At temperatures above 50°C, asbestos-like crystals slowly evaporate, forming SO 3 vapors.
Molecules of sulfur trioxide in the gas phase, in liquid and in alpha crystals have an electronic structure
The molecule has a planar structure with the same bond length (143 pm) as in the sulfur dioxide molecule.
The properties of sulfur trioxide can be largely explained by the lower stability of the sulfur-oxygen double bond compared to the two single bonds between them. So, as a result of the reaction with water, one double bond in sulfur trioxide is replaced by two single bonds in the resulting sulfuric acid.
The increased stability of the product is evidenced by the large amount of heat released during the reaction.
sulfurous acid
A solution of sulfurous acid H 2 SO 3 is obtained by dissolving sulfur dioxide in water. Both sulfurous acid and its salts, sulfites, are strong reducing agents. They form sulfuric acid H 2 SO 4 and sulfates when oxidized by oxygen, halogens, hydrogen peroxide and similar oxidizing agents.
Sulfuric acid has the structure
Sulfuric acid and sulfates
Sulfuric acid H 2 SO 4 is one of the most important chemical products used in the chemical industry and related industries. This is a heavy oily liquid (density 1.838 g cm -3), slightly fuming in air due to the release of traces of sulfur trioxide, which then combine with water vapor to form droplets of sulfuric acid. Pure sulfuric acid, when heated, gives steam rich in sulfur trioxide, and then boils at 338 ° C, maintaining a constant composition (98% H 2 SO 4 and 2% H 2 O). This is the usual industrial "concentrated sulfuric acid".
Concentrated sulfuric acid is highly corrosive. She greedily connects with water; mixing with water is accompanied by the release of a large amount of heat as a result of the formation of a hydronium ion
H 2 SO 4 + 2H 2 O → 2H 3 O + + SO 4 2-
For diluting concentrated sulfuric acid it should be poured into water in a thin stream while stirring the solution; water cannot be added to acid, as this will cause the acid to boil and splatter violently. A dilute acid occupies a smaller volume than its constituents, and the effect of volume reduction is maximum at a ratio of H 2 SO 4: H 2 O =1: 2 [(H 3 O +) 2 (SO 4) 2-].
Chemical Properties and Applications of Sulfuric Acid
The use of sulfuric acid is determined by its chemical properties - it is used as an acid, as a dehydrating agent and an oxidizing agent.
Sulfuric acid has a high boiling point (330°C), which makes it suitable for processing salts of more volatile acids in order to obtain these acids. Nitric acid, for example, can be obtained by heating sodium nitrate with sulfuric acid.
NaNO 3 + H 2 SO 4 → NaHSO 4 + HNO 3
Nitric acid is distilled off at 86°C. Sulfuric acid is also used to make soluble phosphate fertilizers, ammonium sulfate used as a fertilizer, other sulfates, and many chemicals and drugs. Steel is usually derusted by immersion in a sulfuric acid bath ("pickling") before being coated with zinc, tin, or enamel. Sulfuric acid serves as the electrolyte in conventional lead-acid batteries.
Sulfuric acid has such a strong ability to absorb water that it can be used as an effective dehydrating agent. Gases that do not react with sulfuric acid can be dried by passing them through it. The dehydrating power of concentrated sulfuric acid is so great that organic compounds, like sugar, under its action lose hydrogen and oxygen in the form of water.
$C_(12)H_(22)O_(11) \rightarrow 12C + 11H_(2)O$
Sugar (sucrose) H 2 SO 4
Many explosives, such as nitroglycerin, are made by reacting organic compounds with nitric acid to form explosive and water, such as
C 3 H 5 (OH) 3 + 3HNO 3 → C 3 H 5 (NO 3) 3 + 3H 2 O
Glycerin H 2 SO 4 Nitroglycerin
To make these reversible reactions go from left to right, nitric acid is mixed with sulfuric acid, which, due to its dehydrating action, promotes the formation of reaction products. (Two other examples are given in Section 7.7.)
Hot concentrated sulfuric acid is a strong oxidizing agent; its reduction product is sulfur dioxide. Sulfuric acid dissolves copper and can even oxidize carbon
Cu + 2H 2 SO 4 → CuSO 4 + 2H 2 O + SO 2
C + 2H 2 SO 4 → CO 2 + 2H 2 O + 2SO 2
The dissolution of copper in hot concentrated sulfuric acid illustrates the general reaction - dissolution of an inactive metal in an acid with the simultaneous action of an oxidizing agent. Active metals are oxidized to cations under the action of a hydrogen ion, which is then reduced to elemental hydrogen, for example
Zn + 2Н + → Zn 2+ + Н 2 (g.)
A similar reaction with copper does not occur. However, copper can be oxidized to the Cu 2+ ion by the action of a strong oxidizing agent, such as chlorine or nitric acid, or, as shown above, with hot concentrated sulfuric acid.
sulfates
Sulfuric acid combines with bases to form medium sulfates, such as K 2 SO 4 (potassium sulfate), and acid sulfates (sometimes called bisulfates), such as potassium hydrogen sulfate KHSO 4 .
Slightly soluble sulfates are found in the form of minerals, which include CaSO 4 2H 2 O (gypsum), SrSO 4, BaSO 4 (barite) and PbSO 4. Barium sulfate is the least soluble of all sulfates; therefore, its formation in the form of a white precipitate serves as a qualitative reaction to the sulfate ion.
The most common soluble sulfates include: Na 2 SO 4 10H 2 O, (NH 4) 2 SO 4, MgSO 4 7H 2 O (bitter salt), CuSO 4 5H 2 O (copper sulfate), FeSO 4 7H 2 O, (NH 4) 2 Fe (SO 4) 2 6H 2 O (a well-crystallized and easily purified salt used in analytical chemistry for the preparation of standard solutions of ferrous iron), ZnSO 4 7H 2 O, KAl (SO 4) 2 12H 2 O (alum), (NH 4) Al (SO 4) 2 12H 2 O (aluminum-ammonium alum) and KCr (SO 4) 2 12H 2 O (chrome alum).
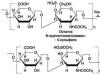
Thio- or sulfonic acids
Sodium thiosulfate Na 2 S 2 O 3 5H 2 O (incorrectly called "sodium hyposulfite") is a substance used in photography. It is obtained by boiling a solution of sodium sulfite with pure sulfur.
SO 3 2- + S → S 2 O 3 2-
Bisulfite ion Thiosulfate ion
Thiosulfuric acid H 2 S 2 O 3 is unstable; when thiosulfate is treated with acid, sulfur dioxide and sulfur are formed.
The structure of the thiosulfate S 2 O 3 2- ion is interesting in that two sulfur atoms are not equivalent. This ion is a sulfate ion SO 4 2-, in which one of the oxygen atoms is replaced by a sulfur atom (Fig. 8.4). The central sulfur atom can be assigned an oxidation state of +6, and the attached sulfur atom can be assigned an oxidation state of -2.
The thiosulfate ion is easily oxidized, especially with iodine, to the tetrathionate ion S 4 O 6 2-
2S 2 O 3 2- → S 4 O 6 2- +2 e
2S 2 O 3 2- + I 2 → S 4 O 6 2- + 2I -
This reaction between thiosulfate ion and iodine is widely used in the quantitative analysis of substances with oxidizing or reducing properties.
Rice. 8.4. Thiosulfate and tetrathionate ions.
Selenium and tellurium
The trans-argonoid compounds of selenium closely resemble the corresponding sulfur compounds. Selenates, salts of selenic acid H 2 SeO 4 are very similar to sulfates. Telluric acid has the formula Te(OH) 6 , and the large central atom has a coordination number of not 4, but 6, just like the iodine atom in the H 5 IO 6 molecule.
Chemistry of the Elements Non-metals of VIA-subgroup
Elements of the VIA subgroup are non-metals, except for Po.
Oxygen is very different from other subgroup elements and plays a special role in chemistry. Therefore, the chemistry of oxygen is highlighted in a separate lecture.
Sulfur is the most important among the other elements. The chemistry of sulfur is very extensive, since sulfur forms a huge variety of compounds. Its compounds are widely used in chemical practice and in various industries. When discussing nonmetals of the VIA subgroup, the greatest attention will be paid to the chemistry of sulfur.
Key Issues Addressed in the Lecture
General characteristics of non-metals of the VIA-subgroup. Natural compounds Sulfur
Simple substance Sulfur compounds
Hydrogen sulfide, sulfides, polysulfides
Sulphur dioxide. Sulfites
Sulfur trioxide
Sulphuric acid. oxidative properties. sulfates
Other sulfur compounds
selenium, tellurium
Simple substances Compounds of selenium and tellurium
Selenides and tellurides
Se and Te compounds in oxidation state (+4)
Selenic and telluric acids. oxidative properties.

Elements of the VIA subgroup |
|||||||||
general characteristics |
|||||||||
The p-elements belong to the VIA subgroup: acid- |
|||||||||
genus O, sulfur S, selenium Se, tellurium Te, polonium Po. |
|||||||||
The general formula for valence electrons |
|||||||||
thrones - ns 2 np 4 . |
|||||||||
oxygen |
|||||||||
Oxygen, sulfur, selenium and tellurium are non-metals. |
|||||||||
They are often grouped under the common name "chalcogens", |
|||||||||
which means "forming ores". Indeed many |
|||||||||
metals are found in nature in the form of oxides and sulfides; |
|||||||||
in sulfide ores |
in small quantities with |
||||||||
there are selenides and tellurides. |
|||||||||
Polonium is a very rare radioactive element that |
|||||||||
which is a metal. |
|||||||||
molybdenum |
|||||||||
To create a stable eight-electron |
|||||||||
chalcogen atoms lack only two electro- |
|||||||||
new The minimum oxidation state (–2) is |
|||||||||
tungsten |
resistant to all elements. It is this degree of oxidation |
||||||||
elements show in natural compounds - ok- |
|||||||||
sides, sulfides, selenides and tellurides. |
|||||||||
All elements of the VIA-subgroup, except for O, exhibit |
|||||||||
seaborgium |
positive oxidation states +6 and +4. Most- |
||||||||
the highest oxidation state of oxygen is +2, it exhibits |
|||||||||
only in conjunction with F. |
|||||||||

The most characteristic oxidation states for S, Se, Te are
xia: (–2), 0, +4, +6, for oxygen: (–2), (–1), 0.
In the transition from S to Te, the stability of the highest oxidation state is +6
decreases, and the stability of the +4 oxidation state increases.
For Se, Te, Po, - the most stable oxidation state is +4.
Some characteristics of atoms of elements ViB - subgroups
Relative |
First energy |
|||
elektrootri- |
ionization, |
|||
value |
kJ/mol |
|||
(according to Polling) |
||||
an increase in the number of |
||||
throne layers; |
||||
an increase in the size of an atom; |
||||
decrease in energy io- |
||||
decrease in electrical |
||||
values |
As can be seen from the above data , oxygen is very different from other elements of the subgroup high value of ionization energy, ma-
large orbital radius of the atom and high electronegativity, only F has a higher electronegativity.
Oxygen, which plays a very special role in chemistry, was considered from
sensibly. Among the other elements of the VIA group, sulfur is the most important.

Sulfur forms a very large number of various |
|||
different connections. Its compounds are known from almost all |
|||
mi elements, except for Au, Pt, I and noble gases. Cro- |
|||
me of widespread compounds S in powers |
|||
3s2 3p4 |
|||
oxidation (–2), +4, +6, are known, as a rule, |
|||
stable compounds in oxidation states: +1 (S2 O), +2 |
|||
(SF2 , SCl2 ), +3 (S2 O3 , H2 S2 O4 ). The variety of sulfur compounds is also confirmed by the fact that only about 20 oxygen-containing acids S are known.
The strength of the bond between S atoms turns out to be commensurate with the
bonds S with other non-metals: O, H, Cl, therefore, S is characterized by
including the very common mineral pyrite, FeS2, and polythionic acids (eg H2 S4 O6 ). Thus, the chemistry of sulfur is quite extensive.
The most important sulfur compounds used in industry
The most widely used sulfur compound in industry and the laboratory is sulfuric acid. The world volume of production of ser-
acid is 136 million tons. (no other acid is produced in such large quantities). Common compounds include
whether sulfuric acid - sulfates, as well as salts of sulfurous acid - sulfites.
natural sulfides are used to obtain the most important non-ferrous metals
thalls: Cu, Zn, Pb, Ni, Co, etc. Other common sulfur compounds include: hydrosulfide acid H2 S, di- and trioxides of sulfur: SO2
and SO3, thiosulfate Na2 S2 O3 ; acids: disulfuric (pyrosulfuric) H2 S2 O7, perox-

sodisulfate H2 S2 O8 and peroxodisulfates (persulphates): Na2 S2 O8 and
(NH4 )2 S2 O8 .
Sulfur in nature
tea in the form of a simple substance, forming large underground deposits,
and in the form of sulfide and sulfate minerals , as well as in the form of compounds,
which are impurities in coal and oil. Coal and oil are obtained as a result of
those decompositions of organic substances, and sulfur is a part of animals and plants
body proteins. Therefore, when coal and oil are burned, sulfur oxides are formed,
polluting the environment.
Natural sulfur compounds
Rice. Pyrite FeS2 is the main mineral used to produce sulfuric acid.
native sulfur;
sulfide minerals:
FeS2 - pyrite or iron pyrite
FeCuS2 - chalcopyrite (copper quanti-
FeAsS - arsenopyrite
PbS - galena or lead luster
ZnS - sphalerite or zinc blende
HgS - cinnabar
Cu2 S- chalcocite or copper luster
Ag2 S - argentite or silver sheen
MoS2 - molybdenite
Sb2 S3 - stibnite or antimony shine
As4 S4 - realgar;

sulfates:
Na2 SO4 . 10 H2 O - mirabilite
CaSO4 . 2H2 O - gypsum
CaSO4 - anhydrite
BaSObarite or heavy spar
SrSO4 is celestine.
Rice. Gypsum CaSO4. 2H2O
simple substance
In a simple substance, sulfur atoms are bonded with two neighboring ones.
The most stable is the structure consisting of eight sulfur atoms,
united in a corrugated ring resembling a crown. There are several modifications of sulfur: rhombic sulfur, monoclinic and plastic sulfur. At ordinary temperature, sulfur is in the form of yellow brittle crystals.
rhombic shape (-S), formed by
ionic molecules S8 . Another modification - monoclinic sulfur (-S) also consists of eight-membered rings, but differs in location
arrangement of S8 molecules in the crystal. When dis-
melting sulfur rings are torn. At the same time, mo-
tangled threads can form, which
Rice. Sulfur
make the melt viscous, with further
As the temperature rises, the polymer chains can break down and the viscosity will decrease. Plastic sulfur is formed during the sharp cooling of the molten
sulfur and consists of entangled chains. Over time (within a few days), it will be converted into rhombic sulfur.
Sulfur boils at 445o C. Equilibria take place in sulfur vapor:
450 o C |
650 o C |
900 o C |
1500 o C |
S 8 S 6 |
S 4 |
S 2 |
S |
S2 molecules have a structure similar to O2.
Sulfur can be oxidized (usually to SO2) and can be reduced
upgraded to S(-2). At ordinary temperatures, almost all reactions involving solid sulfur are inhibited; only reactions with fluorine, chlorine, and mercury proceed.
This reaction is used to bind the smallest droplets of spilled mercury.
Liquid and vaporous sulfur are highly reactive . Sulfur vapor burns Zn, Fe, Cu. When passing H 2 over molten sulfur is formed
H 2 S. In reactions with hydrogen and metals, sulfur acts as an oxidizing
Sulfur can be easily oxidized under the action of halogens.
and oxygen. When heated in air, sulfur burns with a blue flame, oxidizing
up to SO2.
S + O2 = SO2
Sulfur is oxidized with concentrated sulfuric and nitric acids:
S + 2H2 SO4 (conc.) = 3SO2 + 2H2 O,
S + 6HNO3 (conc.) = H2 SO4 + 6 NO2 + 2H2 O
In hot alkali solutions, sulfur disproportionates.
3S + 6 NaOH = 2 Na2 S + Na2 SO3 + 3 H2 O.
When sulfur reacts with a solution of ammonium sulfide, yellow-red polysulfide ions(–S–S–)n or Sn 2– .
When sulfur is heated with a solution of sulfite, thiosulfate is obtained, and
when heated with a solution of cyanide - thiocyanate:
S + Na 2 SO3 = Na2 S2 O3, S + KCN = KSCN

Potassium thiocyanate or thiocyanate is used for the analytical detection of Fe3+ ions:
3+ + SCN – = 2+ + H2O
The resulting complex compound has a blood-red color,
even at a low concentration of hydrated Fe3+ ions in the
About 33 million tons of native sulfur are mined annually in the world. The main amount of extracted sulfur is processed into sulfuric acid and used
used in the rubber industry for the vulcanization of rubber. Add sulfur
binds to double bonds of rubber macromolecules, forming disulfide bridges
ki -S- S-, thereby, as if "stitching" them, which gives the rubber strength and elasticity. When a large amount of sulfur is introduced into rubber, ebo-
nit, which is a good insulating material used in electrical engineering. Sulfur is also used in pharmaceuticals to make skin ointments and in agriculture to control plant pests.
Sulfur compounds
Hydrogen sulfide, sulfides, polysulfides
Hydrogen sulfide H 2 S occurs naturally in sulfuric mineral waters,
present in volcanic and natural gas, formed during the decay of white
kov bodies.
Hydrogen sulfide is a colorless gas with a rotten egg odor and is highly toxic.
It is slightly soluble in water; at room temperature, three volumes of gaseous H2 S dissolve in one volume of water. The concentration of H 2 S in saturated
nom solution is ~ 0.1 mol/l . When dissolved in water, it forms
hydrosulfide acid, which is one of the weakest acids:
H2 S H+ + HS – , K1 = 6. 10 –8 , |
|||||||||||||||||||||||||||||||||||||||||||||||
HS - H+ + S 2–, |
K2 = 1.10 –14 |
||||||||||||||||||||||||||||||||||||||||||||||
Executor: |
Many natural sulfides are known (see the list of sulfide minerals). Sulfides of many heavy non-ferrous metals (Cu, Zn, Pb, Ni, Co, Cd, Mo) are are industrially important ores. They are converted into oxides by firing in air, for example, 2 ZnS + 3 O2 = 2 ZnO + 2 SO2 then the oxides are most often reduced with coal: ZnO + C = Zn + CO Sometimes oxides are brought into solution by the action of an acid, and then the solution is subjected to electrolysis in order to reduce the metal. Sulfides of alkali and alkaline earth metals are practically chemically ionic compounds. Sulfides of other metals - the advantage vein-covalent compounds, as a rule, of non-stoichiometric composition. Many nonmetals also form covalent sulfides: B, C, Si, Ge, P, As, Sb. Natural sulfides As and Sb are known. Sulfides of alkali and alkaline earth metals, as well as sulfides ammonium feed is highly soluble in water, the rest of the sulfides are insoluble rhymes. They are isolated from solutions in the form of characteristically colored precipitates, For example, Pb(NO3 )2 + Na2 S = PbS (t.) + 2 NaNO3 This reaction is used to detect H2 S and S2– in solution.
Some of the water-insoluble sulfides can be brought into solution by acids, due to the formation of a very weak and volatile hydrosulphuric acid. native acid, for example, NiS + H2SO4 = H2S + NiSO4 Sulfides can be dissolved in acids: FeS, NiS, CoS, MnS, ZnS. Metal sulfides and PR values
Sulfides, characterized by a very low value of the solubility product, cannot dissolve in acids with the formation of H2 S. In ki- sulfides do not dissolve in slots: CuS, PbS, Ag2 S, HgS, SnS, Bi2 S3, Sb2 S3, Sb2 S5, CdS, As2 S3, As2 S5, SnS2. If the reaction of dissolution of sulfide due to the formation of H2 S is impossible, then it can be transferred into a solution by the action of concentrated nitric acid slots or aqua regia. CuS + 8HNO3 = CuSO4 + 8NO2 + 4H2O The sulfide anion S 2– is a strong proton acceptor (os- innovation according to Brønsted). So highly soluble sulfides | ||||||||||||||||||||||||||||||||||||||||||||||
§8 Elements VI And the groups.
Oxygen, sulfur, selenium, tellurium, polonium.
General information of elements VI A group:
Group VI A elements (except polonium) are called chalcogenides. There are six valence electrons (ns2 np4) on the outer electronic level of these elements, therefore they show valency 2 in the normal state, and -4 or 6 in the excited state (except for oxygen). The oxygen atom differs from the atoms of other elements of the subgroup by the absence of a d-sublevel in the outer electron layer, which causes high energy costs for the “pairing” of its electrons, which are not compensated by the energy of the formation of new covalent bonds. Therefore, the covalence of oxygen is two. However, in some cases, the oxygen atom, which has unshared electron pairs, can act as an electron donor and form additional covalent bonds according to the donor-acceptor mechanism.
The electronegativity of these elements gradually decreases in the order O-S-Se-Te-Rho. The degree of oxidation is from -2, +2, +4, +6. The radius of the atom increases, which weakens the non-metallic properties of the elements.
The elements of this subgroup form compounds of the form H2 R with hydrogen (H2 O, H2 S, H2 Se, H2 Te, H2 Po). These compounds, dissolving in water, form acids. Acid properties increase in the direction H2 O→H2 S→H2 Se→H2 Te→H2 Po. S, Se and Te form compounds of the RO2 and RO3 type with oxygen. From these oxides, acids of the type H2 RO3 and H2 RO4 are formed. As the atomic number increases, the strength of the acids decreases. All of them have oxidizing properties. Acids like H2 RO3 also exhibit reducing properties.
Oxygen
Natural compounds and preparations: Oxygen is the most abundant element in the earth's crust. In a free state, it is found in atmospheric air (21%); in a bound form, it is part of water (88.9%), minerals, rocks and all substances from which plant and animal organisms are built. Atmospheric air is a mixture of many gases, the main part of which is nitrogen and oxygen, and a small amount of noble gases, carbon dioxide and water vapor. Carbon dioxide is formed in nature during the combustion of wood, coal and other fuels, the respiration of animals, and decay. In some parts of the world, CO2 is released into the air through volcanic activity and also from underground sources.
Natural oxygen consists of three stable isotopes: 816 O (99.75%), 817 O (0.04), 818 O (0.20). The isotopes 814 O, 815 O, 819 O were also obtained artificially.
Oxygen was first obtained in pure form by K.W. Scheele in 1772, and then in 1774 by D.Yu. Priestley, who isolated it from HgO. However, Priestley did not know that the gas he received was part of the air. Only a few years later, Lavoisier, who studied the properties of this gas in detail, established that it is the main part of the air.
In the laboratory, oxygen is obtained by the following methods:
E
water electrolysis. To increase the electrical conductivity of water, an alkali solution (usually 30% KOH) or alkali metal sulfates is added to it:
In general: 2H2 O → 2H2 + O2
At the cathode: 4H2 O+4e¯ → 2H2 +4OH¯
At the anode: 4OH−4е→2H2 О+О2
- Decomposition of oxygen-containing compounds:
Thermal decomposition of Bertolet's salt under the action of MnO2 catalyst.
KClO3 →2KCl+3O2
Thermal decomposition of potassium permanganate
KMnO4 → K2 MnO4 + MnO2 + O2.
Thermal decomposition of alkali metal nitrates:
2KNO3 →2KNO2 +О2.
Decomposition of peroxides:
2H2 O2 → 2H2 O + O2.
2ВаО2 → 2ВаО+О2.
Thermal decomposition of mercury oxide (II):
2HgO→2HgO+О2.
The interaction of peroxides of alkali metals with carbon monoxide (IV):
2Na2 O2 + 2CO2 → 2Na2 CO3 + O2.
Thermal decomposition of bleach in the presence of a catalyst - cobalt salts:
2Ca(OCl)Cl → 2CaCl2 + O2.
Oxidation of hydrogen peroxide with potassium permanganate in an acidic medium:
2KMnO4 + H2 SO4 + 5H2 O2 → K2 SO4 + 2Mn SO4 + 8H2 O + 5O2.
In industry: At present, oxygen is produced in industry by fractional distillation of liquid air. With weak heating of liquid air, nitrogen is first separated from it (tboil (N2) = -196ºC), then oxygen is released (tboil (O2) = -183ºС).
The oxygen obtained by this method contains nitrogen impurities. Therefore, to obtain pure oxygen, the resulting mixture is re-distilled and ultimately 99.5% oxygen is obtained. In addition, some oxygen is obtained by electrolysis of water. The electrolyte is a 30% KOH solution.
Oxygen is usually stored in blue cylinders at a pressure of 15 MPa.
Physicochemical characteristics: Oxygen is a colorless, odorless, tasteless gas, slightly heavier than air, slightly soluble in water. Oxygen at a pressure of 0.1 MPa and a temperature of -183ºС passes into a liquid state, at -219ºС it freezes. In the liquid and solid state, it is attracted by a magnet.
According to the method of valence bonds, the structure of the oxygen molecule, represented by the scheme -:Ö::Ö: , does not explain the great strength of a molecule that has paramagnetic properties, that is, unpaired electrons in the normal state.
As a result of the bonding of the electrons of two atoms, one common electron pair is formed, after which the unpaired electron in each atom forms a mutual bond with an unshared pair of another atom, and a three-electron bond is formed between them. In an excited state, the oxygen molecule exhibits diamagnetic properties, which correspond to the structure according to the scheme: Ö=Ö: ,
Two electrons are missing to fill the electron level in the oxygen atom. Therefore, oxygen in chemical reactions can easily add two electrons and exhibit an oxidation state of -2. Oxygen only in compounds with a more electronegative element fluorine exhibits the oxidation state +1 and +2: O2 F2, OF2.
Oxygen is a strong oxidizing agent. It does not interact only with heavy inert gases (Kr, Xe, He, Rn), with gold and platinum. The oxides of these elements are formed in other ways. Oxygen is included in the reactions of combustion, oxidation, both with simple substances and with complex ones. When non-metals interact with oxygen, acidic or salt-forming oxides are formed, and when metals interact, amphoteric or mixed oxides are formed. Thus, oxygen reacts with phosphorus at a temperature of ~ 60 ° C,
4P+5O2 → 2P2 O5
With metals - oxides of the corresponding metals
4Al + 3O2 → 2Al2O3
3Fe + 2O2 → Fe3O4
when alkali metals are heated in dry air, only lithium forms oxide Li2 O, and the rest are peroxides and superoxides:
2Na+O2 →Na2 O2 K+O2 →KO2
Oxygen interacts with hydrogen at 300 °C:
2H2 + O2 = 2H2 O.
When interacting with fluorine, it exhibits reducing properties:
O2 + F2 = F2 O2 (in electrical discharge),
with sulfur - at a temperature of about 250 ° C:
Oxygen reacts with graphite at 700 °C
C + O2 = CO2.
The interaction of oxygen with nitrogen begins only at 1200°C or in an electric discharge:
N2 + O22NO - Q.
Oxygen also reacts with many complex compounds, for example, with nitric oxide (II), it reacts even at room temperature:
2NO + O2 = 2NO2.
During the oxidation of hydrogen sulfide, when heated, sulfur is formed, or sulfur oxide (IV), depending on the ratio between oxygen and hydrogen sulfide:
2H2 S + O2 = 2S + 2H2 O
2Н2 S + ЗО2 = 2SO2 + 2Н2 О
In most oxidation reactions involving oxygen, heat and light are released - such processes are called combustion.
Ozone
Ozone-O3 is the second allotropic modification of the element oxygen. The O3 molecule has an angular structure (the angle between the bonds is 116º, the length of the O=O bond, l=0.1278 nm) it is a blue gas. Liquid ozone is dark blue. It is poisonous and explosive especially in liquid and solid state). Ozone is formed in the atmosphere during lightning discharges, and has a specific smell of freshness.
Usually, ozone is produced in ozonizers by passing a quiet electrical discharge through oxygen (the reaction is endothermic and highly reversible; the ozone yield is 5%):
3О22О3 ΔН=-285 kJ. Under laboratory conditions, ozone is obtained by acidifying persulfate with nitric acid.
(NH4)2 S2 O8 →H2 S2 O8 +2NH4+
H2 S2 O8 →2SO2 +O3 +H2O
O3 is formed in low yield as a result of the reaction:
3F2 +H2 O(g)→6HF+O3
O3 is the strongest oxidizing agent, oxidizes all metals (except gold and platinum metals) and most non-metals. It converts lower oxides into higher ones, and metal sulfides into their sulfates. In reactions involving O3, O2 is usually formed, for example:
2Ag+O3 →Ag2 O+O2
PbS+4O3 →PbSO4 +4O2
NH2 +3O3 →HNO2 +H2O
Pb(OH)2 +O3 →PbO2 +H2O+O2
When exposed to O3 on alkali metals, ozonides can be obtained - unstable compounds that decompose:
2KO3 →2KO2 +O2
As a strong oxidizing agent, ozone kills bacteria and is therefore used to disinfect the air. A stable ozone layer is located in the atmosphere at a height of ~22 km. This ozone layer protects the Earth from life-damaging pure ultraviolet radiation.
When ozone interacts with a solution of potassium iodide, iodine is released, while this reaction does not occur with oxygen:
2KI + O3 + H2 O \u003d I2 + 2KOH + O2.
The reaction is often used as a qualitative one for the detection of I- or ozone ions. To do this, starch is added to the solution, which gives a characteristic blue complex with released iodine, and it is also of high quality because ozone does not oxidize Cl - and Br- ions.
Water
Physical and chemical properties of water: Pure water is a colorless, tasteless, odorless, transparent liquid. Density of water at the transition her from a solid to a liquid state does not decrease, as with almost all other substances, but increases.
Water is a familiar and unusual substance. There is no substance on earth that is more important to us than ordinary water, and at the same time there is no other substance whose properties would have as many contradictions and anomalies as its properties.
Almost ¾ of the surface of our planet is occupied by oceans and seas. Solid water - snow and ice - covers 20% of the land. The planet's climate depends on water. Geophysicists say that the Earth would have cooled down long ago and turned into a lifeless piece of stone, if not for water. She has a very high heat capacity. As it heats up, it absorbs heat, and as it cools, it releases it. Terrestrial water both absorbs and returns a lot of heat, thereby equalizing the climate. The Earth is protected from cosmic cold by those molecules that are scattered in the atmosphere - in clouds and in the form of vapors.
Water in physical properties differs significantly from other solvents: At 4ºС, water has a maximum density, and only with further heating does its density decrease. If, with a decrease in temperature and during the transition from a liquid to a solid state, water changed similarly to other substances, then when winter approached, the surface layers of natural waters would cool to 0 ° C and sink to the bottom until the entire mass of the reservoir would acquire a temperature of 0 ° C. The water would freeze, the ice floes would sink to the bottom, and the pond would freeze to its full depth. Many forms of life in water would be impossible. In reality, the cooled layer, which has a lower density, remains on the surface, freezes, and thus protects the underlying layers from cooling.
Water has an abnormally high heat capacity (4.18 J/g∙K), so at night, as well as during the transition from summer to winter, the water cools down slowly. And during the day, or during the transition from winter to summer, it also heats up slowly, thus being the temperature regulator on the globe.
Water in its normal state is a liquid, while H2 S, H2 Se, H2 Te are gases. The temperatures of crystallization and evaporation of water are significantly higher than the corresponding temperatures of these compounds.
Water has a very high dielectric constant (78.5 at 298K).
Water is a good solvent for polar liquids and compounds with ionic bonds; it forms crystalline hydrates with many chemical compounds.
For a long time, the unusual properties of water were a mystery to scientists. They are mainly due to the following reasons:
The polar nature of the molecules;
The presence of unshared electron pairs at the oxygen atom;
Hydrogen bonds.
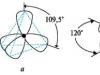
The bond between hydrogen and oxygen atoms is polar, which leads to asymmetry in the distribution of electronic charges and, consequently, to the polarity of the molecule. The bond length is 96 nm, and the angle between bonds is ~ 105º.
The presence of lone pairs of electrons in oxygen and the shift of shared electron pairs from hydrogen atoms to oxygen cause the formation of hydrogen bonds. The binding energy is 25 kJ/mol. The oxygen atom in the water molecule is in a state of sp3 hybridization. Therefore, the HOH bond angle is close to the tetrahedral angle (109.5º).
The molecular weight of vaporous water is 18 and corresponds to its simplest formula. However, the molecular weight of the liquid is higher. This indicates that the association of molecules occurs in the liquid phase; their combination into more complex aggregates, due to the formation of hydrogen bonds between molecules.
In solid water (ice), the oxygen atom of each molecule is involved in the formation of two hydrogen bonds with neighboring water molecules.
The structure of ice belongs to the least dense structures; there are voids in it, the dimensions of which are somewhat larger than the dimensions of a water molecule. When ice melts, its structure is destroyed, but hydrogen bonds remain in the liquid phase, associates are formed, but they exist for a short time: the destruction of some and the formation of other aggregates is constantly occurring. In the voids of such "ice" aggregates, single water molecules can be placed, while the packing of water molecules becomes dense. That is why when ice melts, the volume occupied by water decreases, and its density increases. When water is heated, part of the heat is spent on breaking hydrogen bonds. This explains the high heat capacity of water. Hydrogen bonds between water molecules are completely broken only when water passes into steam.
On Earth, there is one deuterium atom for every 6800 protium atoms, and in interstellar space one deuterium atom is already for 200 protium atoms.
Water is a highly reactive substance.
Water reacts with many metals with hydrogen evolution:
2Na + 2H2 O = H2 + 2NaOH (violently)
2K + 2H2O = H2 + 2KOH (violently)
3Fe + 4H2 O = 4H2 + Fe3 O4 (only when heated)
Not all, but only sufficiently active metals can participate in redox reactions of this type. Alkali and alkaline earth metals react most easily.
From non-metals for example, carbon and its hydrogen compound (methane) react with water. These substances are much less active than metals, but still able to react with water at high temperatures:
C + H2 O ® H2 + CO
CH4 + 2H2 O ® 4H2 + CO2
Water decomposes into hydrogen and oxygen under the action of an electric current. It is also a redox reaction, where water is both an oxidizing agent and a reducing agent:
2H2O ![]() 2H2+O2
2H2+O2
Water reacts with many oxides non-metals. Unlike the previous ones, these reactions are not redox, but compound reactions:
P2 O5 +3H2 O→2H3 PO4 ; N2 O5 +H2 O→2HNO3
Alkali and alkaline earth metal oxides react with water to form the corresponding alkalis:
CaO+H2O→Ca(OH)2
Not all metal oxides are capable of reacting with water. Some of them are practically insoluble in water and therefore do not react with water. These are ZnO, TiO2, Cr2 O3, from which, for example, water-resistant paints are prepared. Iron oxides are also insoluble in water and do not react with it. Many compounds of metals with non-metals easily interact with water to form the corresponding metal hydroxides and hydrogen compounds of non-metals:
PCl3 +3H2O → H3PO3 + 3HCl
Al2 S3 +6H2 O→2Al(OH)3 +3H2 S
Ca3 P2+6H2 O→3Ca(OH)2 +2PH3
Na3 N+3H2 O→3NaOH+NH3
KH+H2O→KOH+H2
Water forms numerous compounds in which its molecule is completely preserved. These are the so-called hydrates. If the hydrate is crystalline, then it is called crystalline hydrate, For example:
CuSO4 +5 H2O→CuSO4 . 5H2O
H2 SO4 + H2 O = H2 SO4 . H2O (sulfuric acid hydrate)
NaOH + H2O = NaOH . H2 O (caustic soda hydrate)
Compounds that bind water into hydrates and crystalline hydrates are used as desiccants. With their help, for example, remove water vapor from moist atmospheric air.
A special reaction of water - photosynthesis - the synthesis of starch (C6 H10 O5) n and other similar compounds (carbohydrates) by plants, occurring with the release of oxygen:
6n CO2 + 5n H2 O = (C6 H10 O5)n + 6n O2 (under the action of light)
Water has catalytic activity. In the absence of traces of moisture, ordinary reactions practically do not occur, for example, sodium, white phosphorus do not oxidize, chlorine does not interact with metals, hydrogen fluoride does not cut glass.
Hydrogen peroxide
Hydrogen peroxide H2 O2 is a hydrogen-oxygen compound containing a record amount of oxygen - 94% by mass. H2O2 molecules contain peroxide groups –О–О–, which largely determine the properties of this compound.
Due to the asymmetric distribution of H-O bonds, the H2O2 molecule is highly polar. A fairly strong hydrogen bond arises between H2O2 molecules, leading to their association. Therefore, under normal conditions, hydrogen peroxide is a pale blue syrupy liquid (density 1.44) with a rather high boiling point (150ºС). When storing H2 O2 decomposes.
Selenium is obtained from waste products of sulfuric acid, pulp and paper production and anode sludge from the electrolytic refining of copper. Selenium is present in sludge along with sulfur, tellurium, heavy and noble metals. To extract selenium, the sludge is filtered and subjected to either oxidative roasting (about 700 °C) or heating with concentrated sulfuric acid. The resulting volatile SeO2 is captured in scrubbers and electrostatic precipitators. From solutions, commercial selenium is precipitated with sulfur dioxide. Sintering of the sludge with soda is also used, followed by leaching of sodium selenate with water and isolation of selenium from the solution. To obtain high-purity selenium used as a semiconductor material, crude selenium is refined by vacuum distillation, recrystallization, and others.
Physical and chemical properties of selenium. The configuration of the outer electron shell of the Se 4s2 4p4 atom; the spins of two p-electrons are paired, while the other two are not paired, so selenium atoms are able to form Se2 molecules or chains of Sen atoms. Chains of selenium atoms can be closed into ring Se8 molecules. The diversity of the molecular structure determines the existence of selenium in various allotropic modifications: amorphous (powdered, colloidal, vitreous) and crystalline (monoclinic α- and β-forms and hexagonal γ-forms). Amorphous (red) powdered and colloidal selenium (density 4.25 g / cm3 at 25 ° C) is obtained by reduction from a solution of selenious acid H2 SeO3, rapid cooling of selenium vapor and other methods. Vitreous (black) selenium (density 4.28 g/cm3 at 25°C) is obtained by heating any modification of selenium above 220°C followed by rapid cooling. Vitreous selenium has a vitreous luster and is brittle. Thermodynamically the most stable is hexagonal (gray) selenium. It is obtained from other forms of selenium by heating to melting with slow cooling to 180-210 ° C and holding at this temperature. Its lattice is built from parallel helical chains of atoms. The atoms within the chains are covalently bonded. All modifications of selenium have photoelectric properties. Hexagonal selenium up to the melting temperature is an impurity semiconductor with hole conductivity. Selenium is a diamagnet (its pairs are paramagnetic).
Selenium is stable in air; oxygen, water, hydrochloric and dilute sulfuric acids do not affect it, it is highly soluble in concentrated nitric acid and aqua regia, it dissolves disproportionately in alkalis:
Se + 4HNO3 → H2 SeO3 + 4NO2 + H2O
3Se + 6KOH → K2SeO3 + 2K2Se + 3H2O
Selenium in compounds has oxidation states -2, +2, +4, +6. With oxygen, selenium forms a number of oxides: SeO, Se2 O3, SeO2, SeO3. The last two are anhydrides of selenous H2 SeO3 and selenic H2 SeO4 acids (salts - selenites and selenates). SeO2 is the most stable. SeO2 and H2 SeO3 with strong oxidizing agents exhibit reducing properties:
3H2 SeO3 + HClO3 → 3H2 SeO4 + HCl
With halogens, selenium gives compounds SeF6, SeF4, SeCl4, SeBr4, Se2 Cl2 and others. Sulfur and tellurium form a continuous series of solid solutions with selenium. With nitrogen, selenium gives Se4 N4, with carbon - CSe2. Compounds with phosphorus P2 Se3, P4 Se3, P2 Se5 are known. Hydrogen interacts with selenium at t>=200 °C, forming H2 Se; H2Se solution in water is called hydroselenic acid. When interacting with metals, selenium forms selenides. Numerous complex compounds of selenium have been obtained. All selenium compounds are poisonous.
Application of selenium . Due to its cheapness and reliability, selenium is used in converter technology in rectifier semiconductor diodes, as well as for photoelectric devices (hexagonal), electrophotographic copiers (amorphous selenium), synthesis of various selenides, as phosphors in television, optical and signal devices, thermistors, etc. n. selenium is widely used to bleach green glass and obtain ruby glasses; in metallurgy - to give cast steel a fine-grained structure, improve the mechanical properties of stainless steels; in the chemical industry - as a catalyst; selenium is also used in the pharmaceutical industry and other industries.
8.4 Tellurium
Natural compounds and obtaining. Basic. sources of tellurium are sludge from electrolytic refining of copper and sludge from sulfuric acid production, as well as alkaline dross from lead refining. During the processing of sulfuric acid sludge by the roasting method (see Selenium), tellurium remains in the cinder, which is leached with hydrochloric acid. Se is precipitated from the hydrochloric acid solution by passing SO2, after which the solution is diluted to an acid content of 10-12% and when heated by the action of SO2, tellurium is precipitated.
During sintering of sludge with soda and subsequent leaching, tellurium passes into a solution and, upon neutralization, precipitates in the form of TeO2. Tellurium is obtained either by direct reduction of TeO2 with coal, or by precipitation by the action of SO2 on hydrochloric acid solutions of TeO2. During the processing of sludge by the sulfide method (leaching with a Na2 S solution), tellurium is isolated from the solution (after Se precipitation by aeration) by the action of dry Na2 S2 O3:
Na2 TeS3 + 2Na2 SO3 → Te + 2Na2 S2 O3 + Na2 S
During the processing of copper electrolyte sludge, tellurium is mainly converted into soda slag, resulting from the remelting of residues into a gold-silver alloy (“Dore metal”). When sulfatization is used, part of the tellurium passes into sulfate solutions together with Cu. Of these, tellurium is precipitated by the action of metallic copper:
H2 TeO3 + 4H2 SO4 + 6Cu → Te + Cu2 Te + 4CuSO4 + 6H2 O
Tellurium is extracted from soda slag after dissolution in water or by neutralization with TeO2 precipitation (it is purified by reprecipitation from sulfide or acid solutions, dissolved in alkali and tellurium is isolated by electrolysis), or rough tellurium is precipitated directly from the soda solution by electrolysis. It is reduced by A1 in an alkaline solution:
6Te + 2A1 + SNaOH → 3Na2 Te2 + 2NaAlO2 + 4H2 O. Then tellurium is precipitated by aeration:
2Na2 Te2 + 2H2 O + O2 → 4Te + 4NaOH
To obtain tellurium of high purity, its volatile compounds are used, in particular TeCl4, which is purified by distillation or rectification and extraction from hydrochloric acid solution. After hydrolysis of TeO2 chloride, H2 is reduced. Sometimes H2 Te is also used for purification. At the final stages of purification, vacuum sublimation, distillation or rectification of tellurium, as well as zone melting or directional crystallization are used.
Physical and chemical properties. Tellurium is a silvery-gray substance with a metallic luster, in thin layers reddish-brown in light, golden yellow in pairs. Tellurium melt above ~ 700 °C has metallic conductivity. Tellurium is diamagnetic, magnetic. susceptibility - 0.31 10-9. Mohs hardness 2.3, Brinell 180-270 MPa; tear resistance 10.8 MPa. Tellurium is brittle and becomes ductile when heated.
For tellurium, the normal electrode potential is 0.56 V. Tellurium, even dispersed, is stable in air, but when heated, it burns (a blue flame with a green halo) to form TeO2. Crystalline tellurium reacts with water above 100°C, amorphous - above 50°C. Concentrated alkali solutions dissolve tellurium to form tellurides and tellurites. Hydrochloric acid and dilute H2 SO4 do not affect tellurium, conc. H2 SO4 dissolves it, the resulting red solutions contain the cation. HNO3 oxidizes tellurium to tellurous acid H2 TeO3 (tellurite salts):
Te + HNO3 → H2 TeO3 + 4NO2 + H2O
Strong oxidizing agents (HClO3, KMnO4, etc.) oxidize to telluric acid H2 TeO4 (tellurate salts):
4Te + 3HClO4 + 4H2O → 4H2 TeO4 + 3HCl
Te + 3H2 O2 → H2 TeO4 + 2H2 O
Tellurium dissolves in solutions of sulfides and polysulfides of alkali metals (with the formation of thiotellurides and thiotellurites). Reacts with Ag salt solutions. Does not dissolve in CS2. It reacts with Cl2, F2 and Br2 at room temperature, with I2 - when heated, alloys with S, P (it does not form compounds), As (giving As2 Te3), with Si (with the formation of Si2 Te3 and SiTe), with Se (forming solid solutions during crystallization). It does not directly interact with boron and carbon; when heated, it forms gaseous unstable TeCO carbonyl. When fused with metals, tellurides are obtained.
Hydrogen telluride H2 Te is a colorless gas with an unpleasant odor; in the liquid state greenish-yellow, crystalline-lemon-yellow; t. kip. - 2°C, so pl. - 51 °С; dense 5.81 g/l; for gas; and in dry air at room temperature it slowly decomposes, in moist air it oxidizes to tellurium; when heated in air, it burns, giving TeO2; solubility in water 0.1 M, aqueous solution-weak acid, K1 2 10-3; strong reducing agent; obtained by the interaction of Al2 Te3 with hydrochloric acid, as well as by electrolysis of a solution of H2 SO4 with a tellurium cathode at 0°C; used to produce high purity tellurium.
TeF6 hexafluoride is a colorless gas; m.p. - 37.8°С, temp. -38.6°C; dense 10.7 g/l; stable in dry air, does not affect glass; dissolves in water, gradually hydrolyzing with the formation of fluorotelluric acids TeFn (OH) 6-n, where n is from 1 to 4, and ultimately telluric acid; forms compounds with metal fluorides, for example. Ag and Ba; obtained by fluorination of tellurium when heated. Tetrafluoride TeF4 - orthorhombic crystals; m.p. 129.6°С, b.p. 194°C (with decomposition); density 4.22 g/cm3; very hygroscopic, easily hydrolyzed; with alkali metal fluorides forms pentafluorotellurates M; obtained by the action of SeF4 on TeO2. Fluorides tellurafluorinating agents.
TeCl4 tetrachloride - yellow crystals; m.p. 224°С, b.p. 381.8°C; dense 3.01 g/cm3; ur-tion of the temperature dependence of vapor pressure \ gp (mm Hg) \u003d 8.791 - - 3941 / T (497 - 653); very hygroscopic, hydrolyzes with water; in concentrated HC1 solution, forming chlorotelluric acid H2 TeC16; from hydrochloric acid solutions it is extracted with tributyl phosphate and other organic solvents; with alkali metal chlorides it forms hexa-M2 [TeCl6] and pentachlorotellurates M[TeC15], with chlorides of Al, Fe(III), Zr and other complexes with cations, for example, TeC13; obtained by chlorination of tellurium; TeCl4 is the starting material for the production of high purity tellurium. Brown TeCl2 dichloride is stable in vapors and can be condensed into a liquid. Two crystalline lower chlorides were also obtained - silver-gray Te2 Cl3 and metastable black Te2 Cl with a metallic sheen.
TeS2 and TeS3 sulfides, which decompose when heated, can be obtained by precipitation from aqueous solutions; TeS7 and Te7 S10 are known. Thiotellurates (eg, Na2 TeS3) can be obtained by dissolving tellurium in a solution of alkali metal polysulfides or sulfur in solutions of polytellurides, as well as by fusion. Thiotellurates are intermediates in some tellurium recovery processes.
Application. The most important field of application of tellurium is the synthesis of the decomposition of tellurides with semiconductor properties. Tellurium is also used in metallurgy for alloying cast iron and steel, Pb, Cu (to increase their mechanical and chemical resistance). Tellurium and its compounds are used in the production of catalysts, spec. glasses, insecticides, herbicides, etc.
Polonium
Natural compounds and obtaining polonium. A radioactive chemical element of group VI of the periodic system, an analogue of tellurium. Atomic number 84. Has no stable isotopes. There are 27 known radioactive isotopes of polonium with mass numbers from 192 to 218, of which seven (with mass numbers from 210 to 218) are found in nature in very small quantities as members of the radioactive series of uranium, thorium and actinium, the remaining isotopes were obtained artificially. The longest-lived isotopes of polonium are artificially produced 209 Rho ( t 1/2 = 102 years) and 208 Rho ( t 1/2 \u003d 2.9 years), as well as 210 Rho contained in radium-uranium ores ( t 1/2 = 138.4 days). The content of 210 Rho in the earth's crust is only 2 10–14%; 1 ton of natural uranium contains 0.34 g of radium and fractions of a milligram of polonium-210. The shortest-lived known isotope of polonium is 213 Po ( t 1/2 = 3 10–7 s). The lightest isotopes of polonium are pure alpha emitters, while the heavier isotopes simultaneously emit alpha and gamma rays. Some isotopes decay by electron capture, and the heaviest ones also exhibit very weak beta activity. Different isotopes of polonium have historical names adopted as early as the beginning of the 20th century, when they were obtained as a result of a chain of decays from the "parent element": RaF (210 Po), AcC "(211 Po), ThC" (212 Po), RaC " (214 Po), AcA (215 Po), ThA (216 Po), RaA (218 Po).
Polonium-210 is synthesized by neutron irradiation of natural bismuth (it contains only 208 Bi) in nuclear reactors (the beta-active isotope of bismuth-210 is formed intermediately): 208 Bi + n → 210 Bi → 210 Po + e. When bismuth is irradiated with accelerated protons, polonium-208 is formed, it is separated from bismuth by sublimation in a vacuum - as M. Curie did. In the USSR, Zinaida Vasilievna Ershova (1905–1995) developed the method for isolating polonium. In 1937 she was sent to Paris to the Institute of Radium in the laboratory of M.Curie (headed at that time by Irene Joliot-Curie). As a result of this business trip, colleagues began to call her "Russian Madame Curie." Under the scientific guidance of Z.V. Ershova, a permanent, environmentally friendly production of polonium was created in the country, which made it possible to implement the national program for launching lunar rovers, in which polonium was used as a heat source.
Long-lived isotopes of polonium have not yet received significant practical application due to the complexity of their synthesis. Nuclear reactions can be used to obtain them.
207Pb + 4He® 208Po + 3n,
208 Bi + 1 H® 208 Po + 2n,
208 Bi + 2D® 208 Po + 3n,
208 Bi + 2D® 208 Po + 2n,
where 4 He are alpha particles, 1 H are accelerated protons, 2 D are accelerated deuterons (deuterium nuclei).
properties of polonium. Tellurium already partially exhibits metallic properties, while polonium is a soft silvery-white metal. Due to the strong radioactivity, it glows in the dark and gets very hot, so continuous heat removal is needed. The melting point of polonium is 254 ° C (slightly higher than that of tin), the boiling point is 962 ° C, therefore, even with a slight heating, polonium sublimates. The density of polonium is almost the same as that of copper - 9.4 g/cm3. In chemical research, only polonium-210 is used; longer-lived isotopes are practically not used due to the difficulty of obtaining them with the same chemical properties.
The chemical properties of metallic polonium are close to those of its closest analogue, tellurium; it exhibits oxidation states of –2, +2, +4, +6. In air, polonium slowly oxidizes (quickly when heated to 250 ° C) with the formation of red dioxide PoO2 (when cooled, it becomes yellow as a result of rearrangement of the crystal lattice). Hydrogen sulfide from solutions of polonium salts precipitates black sulfide PoS.
The strong radioactivity of polonium is reflected in the properties of its compounds. So, in dilute hydrochloric acid, polonium slowly dissolves with the formation of pink solutions (the color of Po2+ ions):
Po + 2HCl ® PoCl2 + H2 ,
however, under the action of its own radiation, the dichloride is converted to yellow PoCl4. Dilute nitric acid passivates polonium, while concentrated nitric acid quickly dissolves it:
Po + 8HNO3 → Po(NO3)4 + 4NO2 + 4H2O
With non-metals of group VI, polonium is related by the reaction with hydrogen to form the volatile PoH2 hydride (mp. -35 ° C, b.p. +35 ° C, easily decomposes), the reaction with metals (when heated) to form black solid polonides (Na2Po, MgPo, CaPo, ZnPo, HgPo, PtPo, etc.) and reaction with molten alkalis to form polonides:
3Po + 6NaOH ® 2Na2Po + Na2PoO3 + H2O.
Polonium reacts with chlorine when heated to form bright yellow PoCl4 crystals, red PoBr4 crystals are obtained with bromine, and polonium reacts with iodine already at 40 ° C to form black volatile iodide PoI4. White polonium tetrafluoride PoF4 is also known. When heated, the tetrahalides decompose to form more stable dihalides:
PoCl4 ® PoCl2 + Cl2 .
In solutions, polonium exists in the form of Po2+, Po4+ cations, PoO32–, PoO42– anions, as well as various complex ions, for example, PoCl62–.
The use of polonium Polonium-210 emits alpha rays with an energy of 5.3 MeV, which are decelerated in solid matter, passing only thousandths of a millimeter and giving up their energy in the process. Its lifetime makes it possible to use polonium as an energy source in atomic batteries of spacecraft: only 7.5 g of polonium is enough to obtain a power of 1 kW. In this respect, it is superior to other compact "atomic" energy sources. Such an energy source worked, for example, on Lunokhod-2, heating the equipment during a long moonlit night. Of course, the power of polonium energy sources decreases over time - by half every 4.5 months, but longer-lived polonium isotopes are too expensive. Polonium is also conveniently used to study the effects of alpha radiation on various substances. As an alpha emitter, polonium mixed with beryllium is used to make compact neutron sources:
9 Be + 4 He ® 12 C + n.
Boron can be used instead of beryllium in such sources. In 2004, inspectors from the International Atomic Energy Agency (IAEA) were reported to have discovered a polonium production program in Iran. This led to the suspicion that it could be used in a beryllium source to "start" with the help of neutrons a nuclear chain reaction in uranium, leading to a nuclear explosion.
Polonium, when it enters the body, can be considered one of the most toxic substances: for 210 Rho, the maximum permissible content in the air is only 40 billionths of a microgram per 1 m3 of air, i.e. Polonium is 4 trillion times more toxic than hydrocyanic acid. The alpha particles emitted by the polonium (and to a lesser extent also the gamma rays) cause damage, which destroy tissues and cause malignant tumors. Polonium atoms can be formed in human lungs as a result of the decay of radon gas in them. In addition, metallic polonium is able to easily form the smallest aerosol particles. Therefore, all work with polonium is carried out remotely in sealed boxes.
The discovery of polonium. The existence of an element with the atomic number 84 was predicted by D.I. Mendeleev in 1889 - he called it ditellurium (in Sanskrit - the "second" tellurium) and suggested that its atomic mass would be close to 212. Of course, Mendeleev could not foresee that this element is unstable. Polonium is the first radioactive element, discovered in 1898 by the Curies in search of a source of strong radioactivity in certain minerals. When it turned out that uranium resin ore radiates more strongly than pure uranium, Marie Curie decided to chemically isolate a new radioactive chemical element from this compound. Before that, only two weakly radioactive chemical elements were known - uranium and thorium. Curie began with the traditional qualitative chemical analysis of the mineral according to the standard scheme, which was proposed by the German analytical chemist K.R. Fresenius (1818–1897) as early as 1841 and according to which many generations of students for almost a century and a half determined cations by the so-called "hydrogen sulfide method ". At the beginning she had about 100 g of the mineral; then American geologists gave Pierre Curie another 500 g. Carrying out a systematic analysis, M. Curie each time checked individual fractions (precipitates and solutions) for radioactivity using a sensitive electrometer invented by her husband. Inactive fractions were discarded, active ones were analyzed further. She was assisted by one of the leaders of the chemical workshop at the School of Physics and Industrial Chemistry, Gustav Bemon.
First of all, Curie dissolved the mineral in nitric acid, evaporated the solution to dryness, dissolved the residue in water, and passed a stream of hydrogen sulfide through the solution. At the same time, a precipitate of metal sulfides precipitated; according to the Fresenius method, this precipitate could contain insoluble sulfides of lead, bismuth, copper, arsenic, antimony, and a number of other metals. The precipitate was radioactive, despite the fact that the uranium and thorium remained in solution. She treated the black precipitate with ammonium sulfide to separate the arsenic and antimony, which under these conditions form soluble thiosalts such as (NH4)3 AsS4 and (NH4)3 SbS3. The solution did not detect radioactivity and was discarded. Lead, bismuth and copper sulfides remained in the sediment.
The part of the precipitate that did not dissolve in ammonium sulfide was again dissolved by Curie in nitric acid, sulfuric acid was added to the solution, and it was evaporated on a burner flame until thick white SO3 fumes appeared. Under these conditions, volatile nitric acid is completely removed, and metal nitrates are converted to sulfates. After cooling the mixture and adding cold water, insoluble lead sulfate PbSO4 turned out to be in the precipitate - there was no radioactivity in it. She discarded the precipitate, and added a strong solution of ammonia to the filtered solution. At the same time, a precipitate fell out again, this time - white; it contained a mixture of basic bismuth sulfate (BiO)2 SO4 and bismuth hydroxide Bi(OH)3. In the solution, complex copper ammonia SO4 of bright blue color remained. The white precipitate, unlike the solution, turned out to be highly radioactive. Since the lead and copper had already been separated, the white precipitate contained bismuth and an admixture of the new element.
Curie again converted the white precipitate into dark brown sulfide Bi2 S3, dried it, and heated it in an evacuated ampoule. Bismuth sulfide did not change at the same time (it is resistant to heat and melts only at 685 ° C), however, some vapors were released from the precipitate, which settled in the form of a black film on the cold part of the ampoule. The film was radioactive and apparently contained a new chemical element - an analogue of bismuth in the periodic table. It was polonium - the first discovered radioactive element after uranium and thorium, inscribed in the periodic table (in the same year 1898, radium was discovered, as well as a group of noble gases - neon, krypton and xenon). As it turned out later, polonium easily sublimates when heated - its volatility is about the same as that of zinc.
The Curies were in no hurry to call the black coating on the glass a new element. One radioactivity was not enough. A colleague and friend of Curie, French chemist Eugene Anatole Demarce (1852–1903), a specialist in the field of spectral analysis (he discovered europium in 1901), studied the emission spectrum of black plaque and found no new lines in it that could indicate the presence of a new element. Spectral analysis is one of the most sensitive methods, allowing the detection of many substances in microscopic quantities invisible to the eye. Nevertheless, in an article published on July 18, 1898, the Curies wrote: “We think that the substance we isolated from uranium resin contains a metal that is not yet known, which is analogous to bismuth in analytical properties. If the existence of a new metal is confirmed, we propose to call it polonium, after the birthplace of one of us” (Polonia in Latin - Poland). This is the only case when a new chemical element, not yet identified, has already received a name. However, it was not possible to obtain weight amounts of polonium - there was too little of it in uranium ore (later polonium was obtained artificially). And it was not this element that glorified the Curie spouses, but radium.
Selenium is not widely distributed in nature. The content of selenium in the earth's crust is . Its compounds are found as impurities in natural sulfur compounds with metals and. Therefore, selenium is obtained from waste products generated in the production of sulfuric acid, in the electrolytic refining of copper, and in some other processes.Tellurium is one of the rare elements: its content in the earth's crust is only .
In the free state, selenium, like sulfur, forms several allotropic modifications, of which the most famous are amorphous selenium, which is a red-brown powder, and gray selenium, which forms brittle crystals with a metallic sheen.
Tellurium is also known in the form of an amorphous modification and in the form of light gray crystals with a metallic luster.
Selenium is a typical semiconductor (see § 190). An important property of it as a semiconductor is a sharp increase in electrical conductivity when illuminated. At the boundary of selenium with a metal conductor, a barrier layer is formed - a section of the circuit that can pass electric current in only one direction. In connection with these properties, selenium is used in semiconductor technology for the manufacture of rectifiers and photocells with a barrier layer. Tellurium is also a semiconductor, but its use is more limited. Selenides and tellurides of some metals also have semiconductor properties and are used in electronics. In small amounts, tellurium serves as an alloying addition to lead, improving its mechanical properties.
Hydrogen selenide and hydrogen telluride are colorless gases with a disgusting odor. Their aqueous solutions are acids, the dissociation constants of which are somewhat larger than the dissociation constant of hydrogen sulfide.
Chemically, hydrogen selenide and hydrogen telluride are extremely similar to hydrogen sulfide. Like hydrogen sulfide, they are highly reducing properties. When heated, they both decompose. At the same time, it is less stable than: just as it happens in the series of hydrogen halides, the strength of the molecules decreases during the transition. Salts of hydrogen selenide and hydrogen telluride - selenides and tellurides - are similar to sulfides in terms of solubility in water and acids. By acting on selenides and tellurides with strong acids, hydrogen selenide and hydrogen telluride can be obtained.
When selenium and tellurium are burned in air or in oxygen, dioxides and are obtained, which under normal conditions are in a solid state and are anhydrides of selenous and tellurous acids.
Unlike sulfur dioxide, and exhibit predominantly oxidizing properties, easily recovering to free selenium and tellurium, for example:
By the action of strong oxidizing agents, selenium and tellurium dioxides can be converted into selenic and telluric acids, respectively.